- The journal
- We all know how valuable our Air Jordans are once we pick them up
- Украина #126535155 , Женские кроссовки nike jordan white black pink — цена 2549 грн в каталоге Кроссовки ✓ Купить женские вещи по доступной цене на Шафе , Air Jordan 2 Retro Low Gym Red x Jordan AJ 2 23 Wings T-Shirt
- Sneakers Draked Viola
- air jordan 6 carmine 2021 release date
- nike dunk armor buy , Cut in half: Nike Metcon 9 Review (2024) , AspennigeriaShops
- NBA x Nike Dunk Low 75th Anniversary
- adidas yeezy 700 v3 fade carbon GW1814 fadcar release date
- nike dunk high cargo khaki white
- adidas y 3 qasa high triple black
- kids air jordan
- Aims & Scope
- Editorial Policies and Processes
- Scientific and Methodological Rigor
- Production and Administration
- Committees
- Authors
- Ethical Considerations
- Submit Article
- Archive
- Indexation
- Search
- Contact us
Farm Comunitarios. 15(3):5-16. doi: 10.33620/FC.2173-9218.(2023).21
Pharmacovigilance of vaccines against COVID-19 in community pharmacies. Results after the second dose and comparison between both
INTRODUCTION
Since its inception in 2019 the COVID-19 pandemic has until 19 January 2023 in over 190 countries been responsible for a total of 668,086,462 confirmed cases and 6,731,034 deaths (1). In Spain, the number of confirmed cases as of 13 January 2023 stands at 13,711,251 and the official number of deaths is 117,789 according to the Spanish Ministry of Health (2).
Successive doses of the vaccines already administered to virtually the entire Spanish population (92.6% population >12 years with full regimen, 91.0% ≥60 years with first booster dose and 56.0% ≥60 years with second booster dose, with adapted vaccine) (3) have both very significantly reduced lethality and the number of people with severe or critical illness; and therefore, hospital and intensive care admissions (2,4).
However, the SARS-CoV-2 virus continues to circulate and successive waves continue to infect with new variants. However, the immunity acquired with each vaccine dose is estimated to be effective for a limited period of time, approximately 5-6 months (5,6); which makes it necessary to continue administering new booster doses, especially to the most vulnerable sectors of the population (7).
Health authorities periodically report suspected adverse reactions or events (ARs) to vaccines reported to the National Pharmacovigilance System (FEDRA), which makes it possible to verify their safety after the administration as of 31 December 2022 of almost 111,293,866 doses in Spain (8).
Pharmacovigilance (PV) activities are included among the competences and tasks of the community pharmacist (CP) in Spanish state and autonomous community legislation (9-11). Consequently, during 2021 the Berbés Group in collaboration with the Official Associations of Pharmacists of Ourense and Pontevedra carried out a training campaign among the CPs of both provinces and an awareness campaign aimed at the population attending community pharmacies (CP) to detect and report suspected adverse reactions experienced after administering the first two doses of vaccines. The results were presented at the SEFAC 10th National Congress of Community Pharmacists and won the prize for best research project. The analysis of those corresponding to the first dose were published in the work of Mera-Gallego et al (2023) (12).
This study shows the results corresponding to suspicions of AR detected with the second dose of vaccines and the comparison of results between both doses.
OBJECTIVES
General
To collaborate in the evaluation of the safety of COVID-19 vaccines after administration to patients who subsequently visit community pharmacies.
Specific
- To record and quantify the suspicions of AR detected after the second dose of vaccines.
- To evaluate the consequences in terms of the need for professional care and the impact on daily life.
- To notify these ARs to the Galician Pharmacovigilance Centre.
- To compare the frequency, degree and type of reactivity to the vaccines between the two doses administered to the same patients.
- To study possible relationships between variables.
MATERIAL AND METHODS
Design
A prospective observational quasi-experimental study, performed in CP in the provinces of Ourense and Pontevedra, from February 2021, when the SARS-CoV-2 vaccines began to be administered to the general population, to December 2021.
Methodology
The methodology is reported in detail in the article by Mera-Gallego et al (2023) (12). Follow-up was performed after the second dose of vaccinated persons incorporated into the study, recording the new suspicions of AR experienced and the impact on their daily life.
The same variables were analyzed as in the first part, results were compared between the two doses of vaccine and possible relationships between variables were studied.
The study received a favourable opinion from the Galician Drug Research Ethics Committee (RECm) (Code 2021/007).
Sample size
For the analysis of AR suspicions with the second dose, the sample size was identical to that reported in the aforementioned article. The sample needed for comparison between variables with a power of 80.0% to detect differences in the contrast of the null hypothesis H₀:p1=p2 by means of a McNemar test for two related samples; taking into account a statistical significance level of 5%, and assuming that the proportion in the experimental group can be reduced by about 20%, is calculated as 119 pairs of experimental units.
Presentation of results and statistical analysis
The statistical programme SPSS® 22.0 for Windows® (IBM® New York, USA) was used for data analysis. Qualitative data were expressed as percentages and quantitative data as mean (m) and standard deviation (SD). The chi-square test was used for the analysis of qualitative variables, student t test for quantitative variables with normal distribution, and Mann-Whitney test for quantitative variables with non-normal distribution. The Wilcoxon test was used for the analysis of paired data. Pearson or Spearman correlation analytical techniques will be used to relate quantitative variables. Statistical significance will be set at P<0.05.
RESULTS
Demographic data
A total of ten pharmacies in the province of Pontevedra and two in the province of Ourense participated, incorporating 781 cases of persons vaccinated with the first dose (First D). After the second dose (Second D) there were 88 (11.3%) fewer cases: 43 had received Jcovden® (formerly COVID-19 Vaccine Janssen®), 39 had been infected between doses, one did not want to receive the second dose due to AR of the first dose and five could not be contacted. There were 693 participants, 441 (63.6%) women and 252 (36.4%) men, whose mean age was 57.8 (SD=18.0) (range=18-97).
The diseases they reported were: 219 hypertension, 163 dyslipidemia, 146 neuropsychiatric disorders, 98 other heart diseases, 86 diabetes, 59 chronic obstructive pulmonary disease (COPD)/respiratory problems, 50 thyroid and 315 other health problems. Number of pathologies per participant: two (0.3%) with eight pathologies, two (0.3%) with seven, 11 (1.6%) with six, 21 (3.0%) with five, 71 (10.2%) with four, 88 (12.7%) with three, 102 (14.7%) with two pathologies, 183 (26.4%) with one, 213 (30.7%) with no pathologies. A total of 23 (3.3%) had some acute pathology (unrelated to the first dose) at the time of receiving the vaccine, the most common were: cold/flu (4), vertigo (3), joint inflammation (3) and cold sores (2).
The demographic characteristics of the participants in our study who received the second dose are shown in Table 1. Totals for the first dose and comparisons between the two are included.
Table 1 Demographic characteristics by sex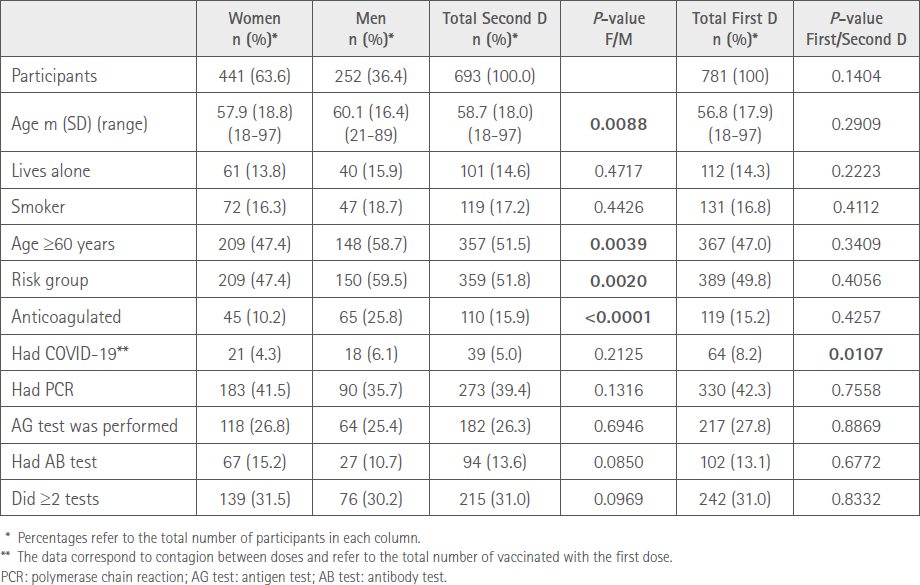
Vaccines
A total of 418 (60.3%), 268 (64.1%) females and 150 (35.9%) males received as second dose Comirnaty® (CO) vaccine, 175 (25.3%) Vaxzevria® (VZ), 66 (37.7%) males and 109 (62.3%) females and 100 (14.4%) Spikevax® (SP), 64 (64.0%) females and 36 (36.0%) males. In the second dose, nine (1.2%) participants received a different vaccine from the first, the first dose of VZ and the second CO (8) and SP (1).
A total of 175 (25.2%) used drugs as prophylaxis for possible AR; 145 (82.9%) used paracetamol.
Suspected adverse reactions
A total of 312 (45.0%) vaccinated, 218 females (49.4%) and 94 (37.3%) males (P<0.01) reported at least one adverse reaction after the second administration; 183 (43.9%) CO, 66 (37.3%) VZ and 63 (63.0%) SP. Table 2 shows the distribution of persons who suffered ARs with the three vaccines according to sex.
Table 2 Distribution by sex and vaccine brand of participants with AR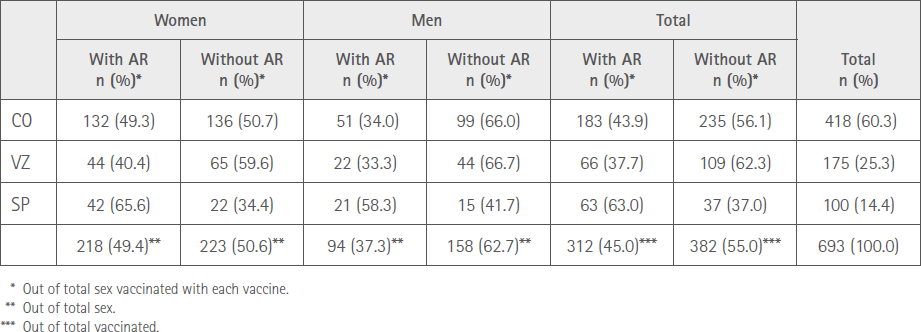
The total number of adverse reactions manifested by respondents was 972; 731 (75.2%) by women and 241 (24.8%) by men, P<0.0001.
The mean number of ARs manifested by vaccinated people was 1.4 (SD=2.2) (range:0-11), 1.0 (SD=1.6) (range:0-10) in males and 1.7 (SD=2.4) (range:0-11) in females. The percentages of vaccinated in relation to the number of ARs referred are shown in Figure 1, with no statistically significant difference between sexes (P=0.1132). The maximum was 11 ARs, in three women.
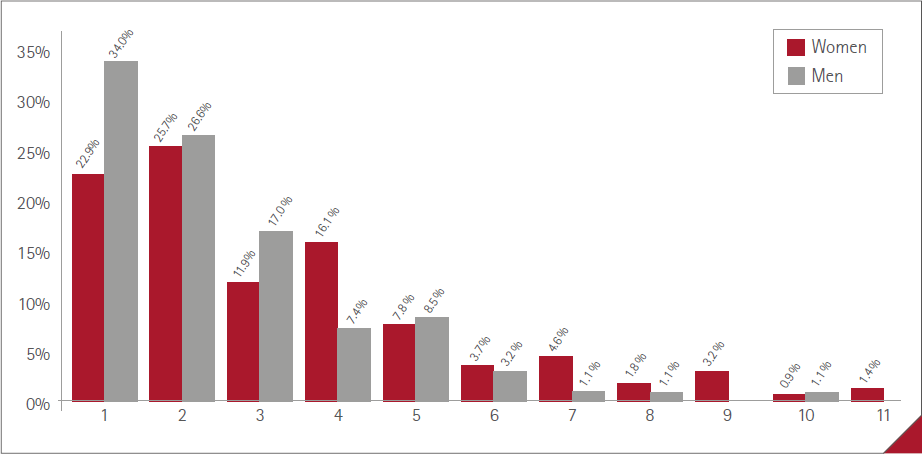
Figure 1 Percentage of participants and number of ARs reported
The most prevalent ARs, affecting >10% of vaccinated people, were: injection site pain 197 (28.4%), tiredness/fatigue 141 (20.3%), muscle pain 111 (16.2%), headache 95 (13.7%) and fever 84 (12.1%).
Table 3 shows all the suspicions of ARs expressed by the subjects vaccinated with the second dose. Of these, we indicate with an * those that had already been detected with the first dose and were not included in the specifications. Two ** indicates those that had not been detected with the first dose and are also ARs not included in the corresponding specifications.
Table 3 Suspected AR affecting those vaccinated with the second dose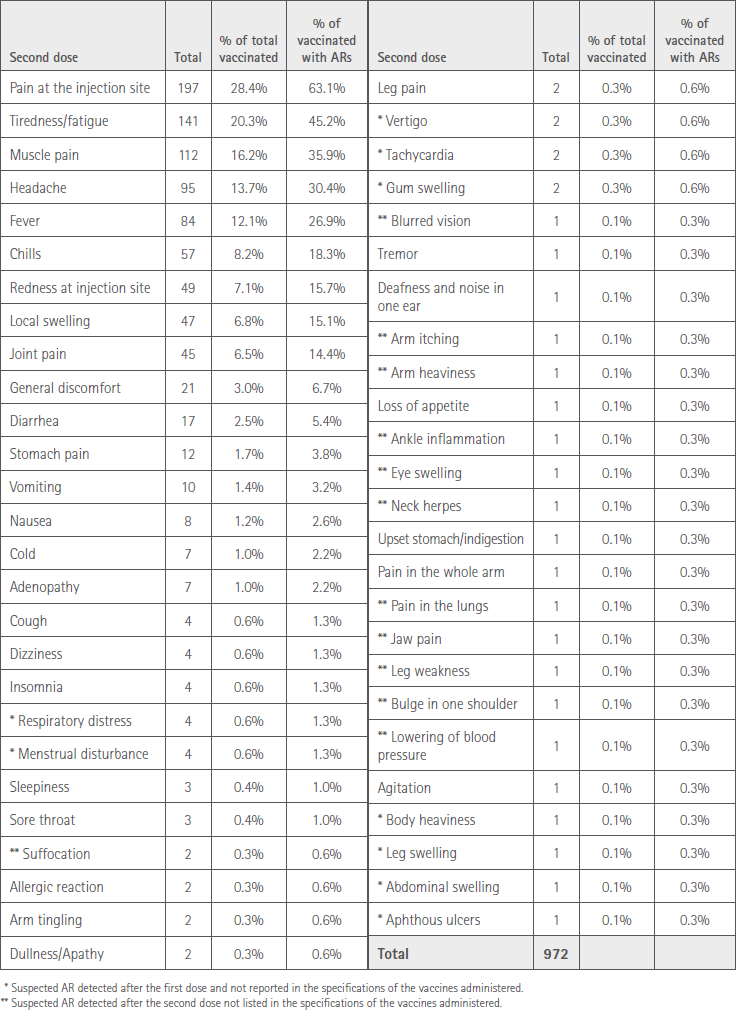
Table 4 shows the percentages of vaccinated people of each sex who reported the most prevalent AR.
Table 4 Number of ARs and type of vaccine by sex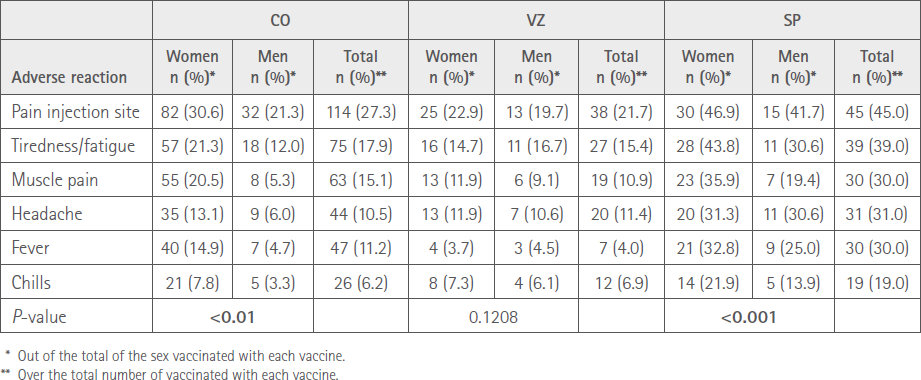
The mean duration of ARs (time from onset to resolution) was less than one day in 71 (7.3%) cases, one to three days in 728 (74.9%), four to five days in 89 (9.2%) and six days or more in 84 (8.6%). No statistically significant difference between women and men, P=0.3963.
The mean duration for each of the three vaccines administered is shown in Figure 2.
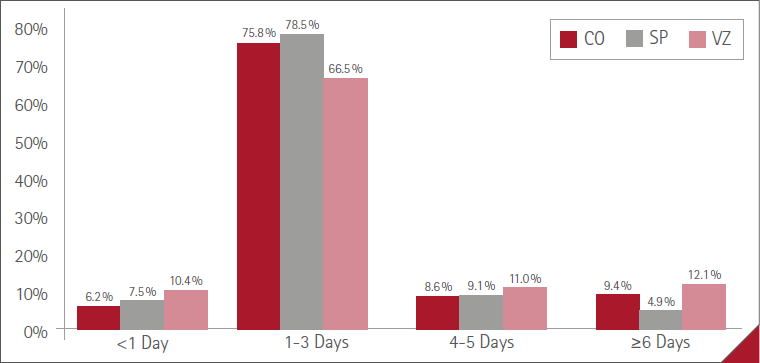
Figure 2 Mean duration of ARs with the three vaccines
Of the 312 respondents who showed reactivity to the vaccine 194 (62.2%) used medication to relieve symptoms, 142 (65.1%) females and 52 (55.3%) males, P<0.001. A total of 176 (90.7%) used paracetamol, as a single drug 161 (83.0%) or together with another medication or active substance 15 (7.7%).
Out of a total of 51 (16.3%) vaccinated, 38 (17.4%) of women with AR and 13 (13.8%) of men (P=0.742) needed professional help: from the primary care physician (PCP) in 10 (19.6%) cases (two men and eight women), 6 (11.8%) (five women and one man) at the casualty department of the point of continuous care (PCC), three (5.9%) women at the hospital (one by referral from the PCP) and 33 (64.7%) (23 women and 10 men) at the pharmacy.
In 292 (93.6%) cases there was spontaneous resolution. However, of the total 70 (10.1%) vaccinated, for 52 (11.8%) women and 18 (7.1%) men, P<0.05, reactivity prevented them from their usual daily activity.
Collaborating pharmacists reported ARs in 201 (64.4%) vaccinated patients to the autonomous PV centre.
Relationships between the variables corresponding to the two doses
Number of vaccinated people with ARs and number of ARs
The number of vaccinated who referred at least one AR was 495 (63.4%) with the first dose and 312 (45.0%) with the second dose, P<0.05. The number of ARs decreased with the second dose, from 1367 (1.8 SD=2.2 per vaccinated person), to 972 (1.2 SD=2.1 per vaccinated person), P<0.05. A total of 227 respondents had AR with both doses, 266 who had AR with the first dose did not have AR with the second dose, and 85 without AR with the first dose had AR with the second dose.
The percentages of vaccinated in regard to the number of ARs manifested by each one are shown in Figure 3. There was no statistically significant difference between the two doses: P=0.4269.
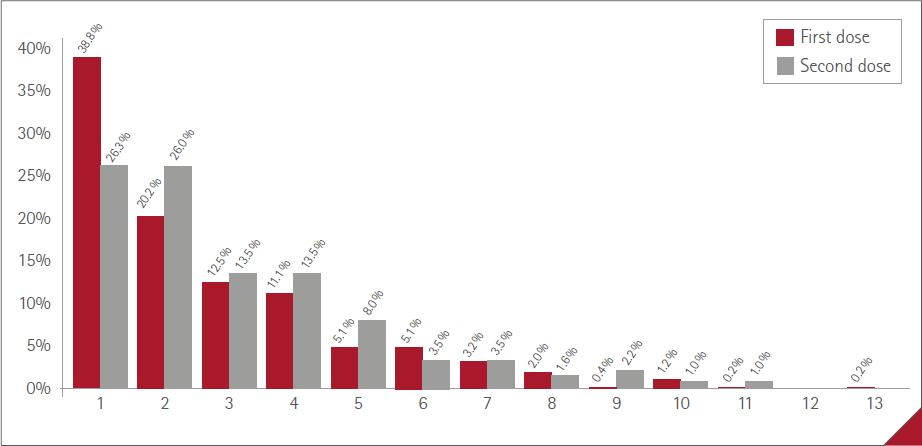
Figure 3 Percentage of vaccinated and number of AR
Table 5 shows the comparison between the first and second doses of the number of ARs according the type of vaccine from the six most prevalent vaccines.
Table 5 Comparison between the first and second dose number of ARs according to vaccine type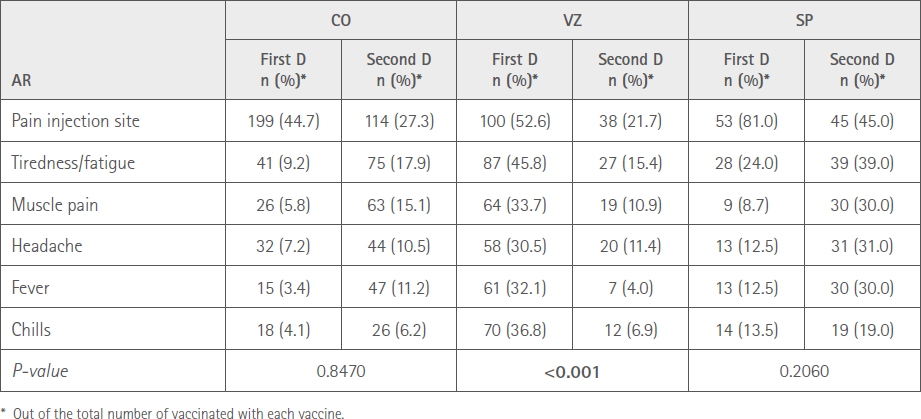
Duration of ARs
The most common mean duration of ARs was one to three days with both doses (73.1% with the first, 74.9% with the second). There were no differences in duration profiles between doses, P=0.7304 (Figure 4).
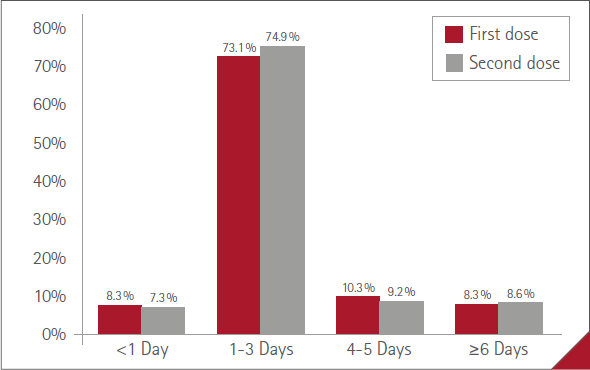
Figure 4 Mean duration of ARs
Differences between sexes
No statistically significant differences between sexes were detected in the number of vaccinated in terms of AR with the first dose. There were statistically significant differences with the second dose, 218 (43.9%) females and 94 (37.1%) males, P<0.05.
No statistically significant differences were detected between sexes with either of the two doses in terms of the need for professional care due to AR or its impact on the daily activity of those vaccinated.
Differences in regard to age:
A statistically significant inverse relationship was found between age and “number of vaccinated with AR”: first dose, mean age of vaccinated with ARs 52.3 (SD=17.3) years; without ARs 64.5 (SD=16.5) years, P<0.05. Second dose, mean age of those vaccinated with ARs 51.1 (SD=17.5); without ARs 63.6 (SD=16.5), P<0.05.
A statistically significant inverse relationship between age and “need for professional care” for ARs with the first dose, mean age of vaccinated who needed care 52.1 (SD=18.3) years; did not need care 57.4 (SD=17.6) years, P<0.05. There was no statistically significant relationship with the second dose.
A statistically significant inverse relationship between age and “prevented daily activity”: first dose, mean age at prevented daily activity 49.3 (SD=16.8) years; did not prevent daily activity 53.3 (SD=17.4) years, P<0.05. Second dose, mean age at prevented daily activity 43.6 (SD=15.8) years, did not prevent daily activity 53.3 (SD=17.4) years, P<0.05.
DISCUSSION
When a drug is marketed, safety studies not only continue but they also intensify, since many adverse reactions, especially those with a lower incidence in the population, are only detected once a large number of patients have used them (13). However, in the case of COVID-19 vaccines, it is also important to differentiate the symptoms presented after administration from those that would correspond to the process of infection by the virus (14), which is why pharmacovigilance is an important activity to be performed in community pharmacies, where patients frequently consult different health problems and are seen by a qualified health professional who can provide adequate guidance and monitor the safety of the vaccines administered in successive doses according to the established vaccination guidelines; and inform the patient of the possible ARs that may occur after administration to avoid possible refusal of subsequent doses.
The possible limitations of the study have already been pointed out in the article cited (12). The subjectivity in the perception of ARs suspicions and their impact, and therefore their manifestation to healthcare professionals or PV services, may be influenced by the good (general case) or bad experience with the first dose. As in most cases the ARs experienced by the participants in our study, although numerous, were generally mild. This could be reflected in the lower number of suspicions manifested before the second dose.
Description of the sample
The number of vaccinated persons who took part in the follow-up of ARs decreased by 11.3% with respect to the first dose, which totalled 693 patients. On the one hand, all patients vaccinated with Jcovden® did not require a second dose to complete the vaccination process. However, those who contracted the infection between both administrations did not receive a second dose during the study period. Only five patients could not be contacted for follow-up of the second dose and one was not vaccinated because he underwent AR with the first dose and did not want to receive the second dose. This indicates that the number of actual losses was very small.
By maintaining virtually the same sample during the first and second doses, the demographic characteristics did not differ between the two phases of the study, with the exception of the data on COVID-19 infection, which was three points higher in patients who had been infected before the first dose, a statistically significant difference that we attribute to the acquisition of a certain degree of immunity even without having completed the vaccination process.
In both phases of the study slightly more than 60% of the sample are women, which coincides with the characteristics of the pharmacy user population (15,16). The mean age of the participants is close to 60 years, similar to that of the participants in other studies (17,18).
We therefore believe that comparison of the results obtained from the analysis of the suspicions of ARs expressed by the vaccinated persons at both vaccination process times can be statistically representative.
There were no major changes between doses in the proportion vaccinated with each of the commercial vaccine brands: Comirnaty® (57/60%), Vaxzevria® (24/25%) and Spikevax® (13/14%). Only 1.2% of participants received a second dose of a vaccine of a different brand than the first, after it had been shown that the combination of different types of vaccines between the first and second doses maintained their effectiveness (18-20). The dissemination in the media of serious ARs, although very low prevalence, of the Vaxzevria® vaccine could have increased the number of people who chose to receive a second dose of another vaccine, since in Galicia this change was allowed, but as can be seen in the results, which we show and analyze in another work (21), this was not the case.
Suspected adverse reactions
The most common suspected adverse reactions were very similar after administration of the first and second doses. Among them, the following stand out: pain at the injection site (which affected almost 30% of those vaccinated), headache, tiredness and fatigue, local swelling, redness, joint pain, chills and fever, which coincide in general terms with the reports of the Spanish Ministry of Health (8) and also with what has been published in other studies (4,17,22-24); although the order of prevalence does not always coincide. The differences can be attributed to the different study methodologies and proportion of the type of vaccine administered, but also, in the case of the Spanish Ministry of Health report, to aggregation of the notification records corresponding to the successive doses up to the date of drawing up the report (16/1/2023) (8).
Both the percentage of vaccinated participants who reported suspected ARs (63%/45%), the number of these (1367/972) and the number of ARs per vaccine (1.8/1.4) decreased significantly after administration of the second dose compared to the first dose. This result also coincides with that of Quiroga et al (25) where 75% of those vaccinated suffered some AR after the first dose, and only 57% of those vaccinated with the second dose, which is not common, as it differs from that found by many other authors (14,17,22-24,26,27), in which the percentage of subjects manifesting ARs increases with successive doses of vaccines.
The highest percentage of vaccinated people with AR was found with Vaxzevria® (83%) in the first dose, followed by Jcovden® (79%), Spikevax® (66%) and Comirnaty® (53%). For the second dose, Spikevax® (63%), followed by Comirnaty® (44%) and Vaxzevria® (38%). Quiroga et al (25) found no differences between the percentage of vaccinated with suspected ARs of Comirnaty® and Spikevax® (75%/74%). However, they conclude, coinciding with other authors, that there are differences according to the technology used, so that, in general, messenger ribonucleic acid (mRNA) vaccines are associated with a higher risk of ARs (23,25,28,29). This is contrary to what was found in our study with the first dose, but coincides with the results of the second dose.
When analyzing the distribution of the six most common ARs in relation to sex and type of vaccine we found that there were significant differences in the number of ARs between sexes for CO and SP, with greater involvement among women. However, after the first dose this difference occurred with VZ (12). When comparing the number of the same six most prevalent ARs between both doses, we observed that, although the percentages are lower in all vaccines with the second dose, they are only significantly lower with VZ, which is consistent with the decreased AR suspicions of this vaccine and with the results already mentioned.
The duration of the discomfort caused by ARs was short, approximately one to three days in 75% of those vaccinated who experienced this, with no differences between men and women, between vaccines or between doses administered. Only 5% of men and 10% of women experienced ARs for more than six days. The short duration of virtually all mild or moderate ARs and their resolution in less than 72 hours coincides with that of numerous studies (8,14,17,23,24), which may have been influenced by treatment with paracetamol (62.2% of those vaccinated with symptoms), partly due to the educational work and indication by the pharmacist.
Impact on health and daily life
Sixty-two percent of respondents who presented a type of AR used medication to alleviate symptoms. Of these, 90% resorted to paracetamol, which although not recommended as prophylaxis prior to vaccine administration because it interferes with the antibody response to some antigens, has been shown to be effective in treating the fever and discomfort accompanying vaccination (30,31).
Despite the fact that most adverse reactions detected are reported in the technical specifications of vaccines and can be deemed mild or moderate reactions, 16% of those vaccinated needed professional help, the same percentage as with the first dose (12). Most (33 of the 51 patients who needed professional help) went to the pharmacy, while for the first dose it had been slightly less than half. On the one hand, and once again, the accessibility of the community pharmacy for the population is clear, and also in our case, we believe that participating in this study, in which the pharmacist was interested in their health in this specific aspect, influenced the fact that this was the professional to whom they turned to in the highest percentage after administration of the second dose.
The need for professional attention, that is, to go to the health centre or PCC, either in consultation at a primary care centre or a primary or hospital casualty department, or to the pharmacist in the community pharmacy, to consult and try to solve the health problems caused by the ARs attributable to the vaccine is an aspect not studied in the literature that we have been able to consult, except in our study (12). The same is true of the impact on daily life tasks, including work activity in active workers. With the first dose, 15% of those vaccinated had their usual daily activity affected, with no differences between sexes (12), while with the second dose this percentage was reduced to 10%. In this case, women were more affected (12%/7%). As this is the same sample (except for those not vaccinated with the second dose), we can attribute this difference not only to the decrease in the number of ARs after the second dose, but again to the health education work carried out in the successive follow-up visits to the pharmacist in the collaborating community pharmacy; and which may have contributed to adequately dimension the significance of the perceived discomfort as suspected ARs from the vaccines.
Relationships with demographic variables
Although in our study with the first dose there were differences, they were not statistically significant. Between the percentage, within each sex, of vaccinated men (59%) and women (66%) who presented adverse reactions, there were differences after the second dose of the vaccine, with significantly more women presenting some AR. This is also reported by the Spanish Ministry of Health, which recorded a percentage of 74% of women among those who reported ARs in the report coinciding in time with the completion of our study (32) and that is virtually maintained (72%) in the current report (8). This is also what occurs in most works consulted (4,17,17,22,28,33).
For both the first and second doses of the vaccine, it was observed that the older the age, the lower the number of vaccinated who report suspicions of ARs. Therefore, the mean age of people without ARs is approximately 64 years, while that of those who report having experienced ARs is 12 years younger. This inverse relationship between age and reactivity to vaccine administration is also found in numerous reports consulted (8,17,27,28,33,34).
Age and sex are also predictive factors (although not significantly in all doses) in terms of the need for professional assistance to treat ARs and impairment in daily life, so that the older the age and for male sex, the lower the risk of requiring professional assistance and the lower the impairment in daily activities. Data that we have not been able to contrast with other studies.
CONCLUSIONS
As in the previous work, which analyzed the suspicions of ARs reported by study participants after administration of the first dose of the vaccine, with the second dose, the number of adverse reactions reported by the vaccinated was high, as was the percentage of vaccinated persons who experienced them; although both were significantly reduced after the second dose.
The most commonly reported suspected ARs coincided for the two doses: local (pain and redness at the injection site and local swelling) and general (chills, fever, malaise and fatigue), nervous system (headache and dizziness) and musculoskeletal system (myalgia and arthralgia) disorders.
Although the ARs experienced were generally mild and resolved within a short period of time, a considerable number of vaccinated people required professional help and had their usual activities affected; in the latter case less so after the second dose.
Recourse to the pharmacy and the community pharmacist for professional help was notable and increased after the second dose, which reveals the importance of the work being performed by the community pharmacy during the pandemic and the importance of programmes such as the one we are analyzing to monitor users’ health problems.
Analysis of the variables collated in regard to the sex of those vaccinated showed that in both doses more women than men experienced ARs and in greater numbers, used medications to alleviate symptoms and were affected in their daily, work and private routines.
In regard to age, the younger age group reported more suspected ARs, and in greater numbers, needed professional care (only with the first dose) and were prevented from performing their daily activities.
In conclusion, we believe that, although the administration of COVID-19 vaccines is associated with a higher risk of adverse effects than recognized in official reports, they are generally mild and of short duration, and their benefits overwhelmingly outweigh them. Community pharmacists should be actively involved in the follow-up of ARs experienced by their patients, collaborating with other primary care professionals, to whom it is still difficult to gain access because the emphasis continues to be on out-of-hospital care.
ACKNOWLEDGMENTS
To the 784 vaccinated patients who agreed to join our project and take part in the subsequent follow-up. To all the community pharmacists in the pharmacies who collaborated in the study. Without the availability, patience and dedication of all of them, it would not have been possible to perform the study.
Collaborating pharmacists: Carballido Pharmacy (Ourense): Rosa Mª Carballido Gago, Mª Pilar González Abades; MT Rodríguez Pharmacy (Ourense): Mª Teresa Rodríguez Rodríguez Rodríguez, Laura León Rodríguez, Rubén Alonso Bailez, Lara Fernández Puga; Rey Carballeda Pharmacy (Pontevedra): Belén Rey Carballeda, Begoña Gago Arca; Andrés Pharmacy (Vigo): J. Carlos Andrés Iglesias, Rocío Mera Gallego, Alex Piñeiro Abad; Colmenero Pharmacy (Vigo): Patricia López Colmenero, Miriam Barreiro Juncal, Laura Pérez Molina; Acea Pharmacy (Vigo): Raquel Acea Lorenzo, Mónica González Blanco; Mallada Pharmacy (Vigo): Álvaro Mallada Garabato, Diego López Cantorna; Fornos Pharmacy (Cangas do Morrazo): José A. Fornos Pérez, Patricia García Rodríguez, Lorena Tenorio Salgueiro; Cordeiro Pharmacy (Moaña): Marta Fernández Cordeiro; Cebreiro Pharmacy (Tui): Mª José Cebreiro Mosquera, Carla Novás García; Rodríguez Pharmacy (Crecente): Ramón Rodríguez Álvarez, Bibiana Guisado Barral, Xurxo Diz Gerpe; Alonso Pharmacy (O Rosal): Ricardo Alonso Pérez, Yésica Oza Araujo.
We would also like to thank the staff of the Technical Department of the Official Association of Pharmacists (OAP) of Pontevedra and the presidents of the OAPs of Pontevedra and Ourense for their support and for disseminating our project among their members.
REFERENCES
1. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). [Consulted 19/1/2023]. Available at: https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6
2. Ministerio de Sanidad. Actualización nº 648. Enfermedad por el coronavirus (COVID-19). 13.01.2023. [Consulted 19/1/2023]. Available at: https://www.sanidad.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_654_COVID-19.pdf
3. Ministerio de Sanidad. Gestión integral de la vacunación COVID-19. Informe de actividad. 13/1/2023. [Consulted 19/1/2023]. Available at: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Informe_GIV_comunicacion_20230113.pdf
4. Graña C, Lina Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database of Systematic Reviews. Dec. 2022; 12:CD015477. doi:10.1002/ 14651858.CD015477
5. Organización Mundial de la Salud (OMS). ¿Cuánto dura la protección de las vacunas contra la COVID-19? Actualizado 17/05/2022. [Consulted 21/1/2023]. Available at: https://www.who.int/es/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines
6. Ferdinands JM, Rao S, Dixon BE, Mitchell, PK, DeSilva Malini B, Irving SA, et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022. Morb Mortal Wkly Rep. 2022;71(7):255–263. http://dx.doi.org/10.15585/mmwr.mm7107e2
7. Xunta de Galicia. Dirección Xeral de Saúde Pública. Plan galego de vacinación fronte ao SARS-Cov-2. Versión 9.4 29/12/2022. [Consulted 24/1/2023]. Available at: https://coronavirus.sergas.gal/Contidos/Documents/1106/PLAN_COVID_VERSION_9.4.pdf
8. Ministerio de Sanidad. 19º Informe de Farmacovigilancia sobre Vacunas COVID-19. 19/1/2023. [Consulted 24/1/2023]. Available at: https://www.aemps.gob.es/informa/19o-informe-de-farmacovigilancia-sobre-vacunascovid-19/
9. Ley 33/2011 de 4 de octubre, General de Salud Pública. Boletín Oficial del Estado, Núm. 240 de 5 de octubre de 2011. Available at: https://www.boe.es/eli/es/l/2011/10/04/33/con
10. Real Decreto 577/2013, de 26 de julio, por el que se regula la farmacovigilancia de medicamentos de uso humano. Ministerio de Sanidad, Servicios Sociales e Igualdad. BOE nº 179, de 27 de julio de 2013. Available at: https://www.boe.es/eli/es/rd/2013/07/26/577/con
11. Ley 3/2019, de 2 de julio, de ordenación farmacéutica de Galicia. Diario Oficial de Galicia, nº 130 de 10 de julio de 2019. Available at: https://www.boe.es/buscar/pdf/2019/BOE-A-2019-13517-consolidado.pdf
12. Mera-Gallego R, León-Rodríguez L, González-Blanco M, Mera-Gallego I, García-Rodríguez P, López-Cantorna D, et al. Farmacovigilancia de las vacunas frente a Covid-19 en farmacias comunitarias. Resultados con la primera dosis. Farm Comunitarios. 2023 Jan 26;15(1):22-40. https://www.doi.org/10.33620/FC.2173-9218.(2023).04
13. Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. BMJ. 1998;316(7140):1295-8. doi:10.1136/bmj. 316.7140.1295
14. Álvarez L, Castiñeiras M, González F, Gonzáles JM, Casma RM, Nuñez MC. Reacciones adversas notificadas tras la administración de vacuna frente a Covid-19 en trabajadores de un hospital terciario. Rev Asoc Esp Med Trab. 2021;30(2):125-261. Available at: https://scielo.isciii.es/scielo.php?script=sci_abstract&pid=S1132- 62552021000200217&lng=es
15. Zalve JL, Gómez A, Martínez R, Pardo E, Córdoba A, Garrido E. Proyecto RUMBO. Estudio de la experiencia de paciente en la farmacia comunitaria española: perfil de los pacientes que acuden a las farmacias comunitarias en España. Farm Comunitarios. 2018:10(Supl.1):334. Available at: https://www.farmaceuticoscomunitarios.org/es/journal-article/proyecto-rumbo-estudio-experiencia-paciente-farmacia-comunitaria-espanola-perfil
16. Mera-Gallego R, León-Rodríguez L, Mera-Gallego I, González-Blanco M, Fernández-Cordeiro M, Piñeiro-Abad A, et al. Percepción de los usuarios de la farmacia comunitaria sobre la COVID-19 al final de la alarma y comparación con la situación al inicio. Farm Comunitarios. 2021 Jan 20;13(1):7-16. doi:10.33620/FC.2173-9218.(2021/Vol13).001.03
17. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021 Jul;21(7):939-949. PMID: 33930320. doi:10.1016/s1473-3099(21)00224-3
18. Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomized study. Lancet 2022;399(10324):521–529. doi:10.1016/S0140-6736(22)00094-0
19. Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al, on behalf of theCombiVacS Study Group. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial, The Lancet, 2021;398(10295):121-130. doi:10.1016/S0140-6736(21)01420-3
20. Campuzano-Bulgarín YF, Villena-Torres CD, Chunga-Campuzano MF, Castro-Ochoa GE. Estudios y resultados sobre la combinación de la inmunización con la vacuna Pfizer y Astrazeneca. Dom Cien. 2021;7(4):1537-1551. doi:10.23857/dc.v7i4
21. Mera-Gallego R, Piñeiro-Abad A, Mera-Gallego I, Andrés-Iglesias JC, Fornos-Pérez JA, Andrés-Rodríguez NF. Farmacovigilancia de las vacunas frente a la COVID-19 en farmacias comunitarias VI. Elección de la segunda dosis. Farm Comunitarios. 2022 Jun 15;14(Supl 1. Congreso SEFAC):95. doi:10.33620/FC.2173-9218.(2022).CMC.99
22. Lounis M, Aouissi HA, Abdelhadi S, Rais MA, Belkessa S, Bencherit D. Short-Term Adverse Effects Following Booster Dose of Inactivated-Virus vs. Adenoviral-Vector COVID-19 Vaccines in Algeria: A Cross-Sectional Study of the General Population. Vaccines. 2022;10(11):1781. doi:10.3390/vaccines10111781
23. Sutton N, San Francisco Ramos A, Beales E, Smith D, Ikram S, Galiza E, et al. Comparing reactogenicity of COVID-19 vaccines: a systematic review and meta-analysis. Expert Rev Vaccines. 2022 Sep;21(9):1301-1318. doi:10.1080/14760584.2022.2098719
24. García Alonso E, Sánchez Peinador C, García Luengo A, Ramos Herranz RA, Garrido Mesa M, Serrano Sanz MR. Análisis de las reacciones adversas a la vacuna contra COVID-19 en la población de la Zona Básica de Salud de Cantalejo. Med Gen Fam. 2022;11(5):197-205. doi:10.24038/mgyf.2022.046
25. Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: Acceptance and side effects. Journal of Healthcare Quality Research. 2021;36(6):236-369. doi:10.1016/j.jhqr.2021.05.002
26. Al Khames QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahha, AT, et al. Safety of COVID-19 vaccines. J Med Virol. 2021;93(12):6588-6594. doi:10.1002/jmv.27214
27. Benitez Fuentes JD, de Luna Aguilar A, Jimenez Ortega AF, Flores Navarro P, Bartolomé Arcilla J, Baos Muñoz E, et al. Adverse drug reactions to the three doses of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) mRNA-1273 vaccine in a cohort of cancer patients under active treatment of a tertiary hospital in Madrid, Spain. F1000Res. 2022 Apr 19;11:434. doi:10.12688/f1000research.110268.2
28. Lounis M, Rais MA, Bencherit D, Aouissi HA, Oudjedi A, Klugarová J, et al. Side Effects of COVID-19 Inactivated Virus vs. Adenoviral Vector Vaccines: Experience of Algerian Healthcare Workers. Front Public Health. 2022;10:896343. doi:10.3389/fpubh.2022.896343
29. Kouhpayeh H, Ansari H. Adverse events following COVID-19 vaccination: A systematic review and meta-analysis. International Immunopharmacology. 2022;109:108906. doi:10.1016/j.intimp. 2022.108906
30. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629. doi:10.1371/journal.pone.0106629
31. Wysocki J, Center KJ, Brzostek J, Majda-Stanislawska E, Szymanski H, Szenborn L, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-35. doi:10.1016/j.vaccine.2017.02.035
32. Ministerio de Sanidad. 11º Informe de Farmacovigilancia sobre Vacunas COVID-19. 20/12/2021. [Consulted 24/1/2023]. Available at: https://www.aemps.gob.es/laAEMPS/docs/informe-farmacovigilancia-diciembre-2021.pdf
33. Alzarea AI, Khan YH, Alatawi AD, Alanazi AS, Alzarea SI, Butt MH, et al. Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile. Vaccines 2022;10:924. doi:10.3390/vaccines10060924
34. Anderson EJ. Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383:2427-38. doi:10.1056/NEJMoa2028436
Cite this article as: Mera-Gallego R, León-Rodríguez L, Barreiro Juncal M, Pérez Molina L, Guisado Barral B, Busto Domínguez I, Andrés-Rodríguez NF, Fornos-Pérez JA. Pharmacovigilance of vaccines against COVID-19 in community pharmacies. Results after the second dose and comparison between both. Farm Comunitarios. 2023 XX XX;15(3):1-12. doi:10.33620/FC.2173-9218.(2023).21
Editor: © SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria.
Copyright© SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria. This article is available from url https://www.farmaceuticoscomunitarios.org/. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en


