- The journal
- nike air force with skinny jeans girls , Manor PHX – Cheap Ietp Jordan Outlet , Premium Footwear & Streetwear Boutique
- adidas superstar heren groen shoes sale india 2018
- In this incredible set of vintage prints is the Air Jordan VIII - Air Jordan Retro 2016 Release Dates - The Air Jordan 1 Mid continues its impressive lineup
- nike air jordan 1 outlet
- Мужские россовки adidas decade hi b - Украина #28353944 , adidas Originals Vit t-shirt i boyfriend-modell med stor logga - ball оригинал кожа — цена 2900 грн в каталоге Кроссовки ✓ Купить мужские вещи по доступной цене на Шафе
- cheap nike sb dunk high new york mets cloud grey rush blue team orange white 2022 for sale dh7155 001
- adidas y 3 qasa high triple black
- Nike Kyrie 8 DC9134 001 Release Date 4
- adidas Ultra Boost 2022 COLD.RDY Magic Grey GZ0128 Release Date
- Travis Scott Fragment Air Jordan 1 Low DM7866 140 Release Date 4
- Aims & Scope
- Editorial Policies and Processes
- Scientific and Methodological Rigor
- Production and Administration
- Committees
- Authors
- Ethical Considerations
- Submit Article
- Archive
- Indexation
- Search
- Contact us
Farm Comunitarios. 2023 Jan 02;15(1):22-40. doi: 10.33620/FC.2173-9218.(2023).04
Pharmacovigilance of vaccines against COVID-19 in community pharmacies. Results with the first dose
INTRODUCTION
The current pandemic called as coronavirus-2019 infectious disease (COVID-19), caused by coronavirus-2 and leads to severe acute respiratory syndrome (SARS-CoV-2). Since its onset there have been 596,211,108 confirmed cases and 6,453,854 deaths at 22 August 2022 in over 190 countries [1]. In Spain the number of confirmed cases and deaths in the report of 19 August 2022 stands at 13,314,764 and 112,128, respectively, according to the Spanish Ministry of Health [2].
After three successive epidemic waves in under one year, without the containment measures having been proven effective the capacity of the Spanish health system has been overwhelmed and unable to react on the scale required.
The availability of suitable vaccines for the population turned into the only way to return to a situation as close to normal as possible.
The Human Use Medicines Committee (CHMP) of the European Medicines Agency (EMA) recommended in December 2020 provisional authorization of Comirnaty® (CV), a COVID-19 vaccine developed by BioNTech and Pfizer, after a favourable evaluation of its risk/benefit ratio [3]. During the subsequent weeks the same outcome was attained after the CHMP evaluated the vaccine from the laboratory Moderna, Spykevax® (SV), Vaxzevria® (VV) from Oxford-Astra-Zeneca, and a little later the COVID-19 Vaccine Janssen® (JV) (currently called Jcovden®), whereby in early 2021 four COVID-19 vaccines were already being administered to the Spanish population [4].
The cumulative experience in vaccine development and the financial and scientific efforts of countries, researchers and the pharmaceutical industry made vaccine availability possible in record time. During phase 2 and 3 - under clinical trials conditions and with larger than usual samples – they have proven their efficacy and safety [5].
Nonetheless, in light of the extended administration to the general population and the study’s short term prior to their marketing, procedures need to be implemented that enable close follow up of their safety, both rare adverse events, undetected in previous trials, and those that might appear in the medium or long term that enable completing their efficacy and safety profile [6]; because it is estimated that during the three phases prior to the marketing of a drug only adverse reactions (ADR) are detected with an incidence higher than 1/250 people. A total of 30,000 people in treatment are needed to detect a patient with an adverse reaction incidence 1/10,000 [7].
The Community Pharmacist (CP) is a healthcare professional able to identify potential new adverse reactions and the community pharmacy is a health space recognized as a private healthcare establishment, of public interest, which remained open during the pandemic and accessible to the population. Pharmacovigilance activities (PV) are among the skills and undertaking of the community pharmacist in state and autonomous legislation [8-10]. There are also references of the CP’s effectiveness in detecting ADR both locally in recently marketed [11] or especially monitored medicines [12]. For example nationally, by means of the creation of sentinel pharmacy networks [13-15], whereby the activity aimed to be performed and documented enables collaborating with pharmacies taking part in the strategy to monitor the safety of COVID-19 vaccines [6,16].
Consequently, the Berbés Group for Research and Teaching, in collaboration with the Official Colleges of Pharmacists of Pontevedra and Ourense implemented a pharmacovigilance project with a group of community pharmacies taking part. The purpose was to record the possible adverse reactions undergone by people vaccinated. This work reports the results corresponding to suspected ADR detected with the first dose of the vaccines.
AIMS
General
Collaborating on the evaluation of COVID-19 vaccine safety after its administration to patients who subsequently visit community pharmacies.
Specific
- Set out the profile of subjects affected by ADR potentially caused by SARS-CoV-2 vaccines.
- Record and quantify the possible ADR detected after administration of the first dose of vaccines.
- Evaluate the need for professional treatment and how daily life is affected.
- Evaluate the CP’s action in the treatment of people with ADR indicating pharmacological and non-pharmacological measures to relieve these.
- Notify these ADR to the Galician Centre for Pharmacovigilance.
MATERIAL AND METHODS
Design
A prospective, observational quasi-experimental study performed in community pharmacies (CPs) from the provinces of Ourense and Pontevedra, since SARS-CoV-2 vaccines started to be administered to the general population as of February to December 2021.
Subjects
All pharmacy users who answered positively when asked whether they received the COVID-19 vaccine, of age, with capacity to understand the questionnaire and who signed the informed consent to take part in the study.
Variables
Primary endpoint
Number and percentage of subjects who show at least one ADR in regard to the number of patients incorporated into the study.
Other endpoints
Demographic characteristics of the subject, age, sex, lives alone or accompanied, place of work, has had the disease (Yes/No), tests performed and results, belongs to an at risk group, pathologies and routine and current medication. Number, type and frequency of suspected reactivity to the vaccine and adverse events observed. Action and consequences of the observed reaction. Impact on their daily life.
Sample Size
Attaining statistical significance of data on ADR observed was not sought. Rather to detect ADR with the purpose of collaborating on setting out their safety profile for rare and medium or long term reactions; whereby the activity was not stopped when the necessary sample size was attained. Rather, this continued over the time incorporated into routine action for dispensing by the community pharmacists taking part.
Nonetheless, to be able to evaluate the significance of results and have an approximate minimal dimension of the study, it was calculated that to attain an accuracy of 5.0% in estimating a proportion by means of a normal, 95% bilateral, asymptotic confidence interval. Assuming a proportion (percentage of subjects who show at least one ADR) of 20.0%, it will be necessary to include 246 subjects in the study. Foreseeing the possibility of 20% losses, defective or incomplete it is foreseen to incorporate into the study at least 307 individuals.
Procedure
After the offer to take part in the study, the interview took place in the personalized treatment area. The subject’s answers were collated in a record log filled in by the pharmacist together with the patient (Apendix 1 as a separate file). The list of possible reactions to the vaccine was based on those declared by manufacturing laboratories; in the papers that reported the results of efficacy and safety trials performed [17-19] and in which their specifications appeared [20-23]. Records in which all the sections could not be filled in were excluded.
In person or telephone follow up was carried out 10 days after the first and second dose of vaccine received and monthly as of the first month after the second dose.
Educational intervention
as a complement to the interview according to the results, an educational interview took place. Non-vaccinated persons were notified about the importance of vaccination with the purpose of delaying the severe consequences of COVID-19 both on an individual and social level, its risks and actual benefits in light of scientific evidence. Those already vaccinated were educated over recognition and action in light of suspected ADR, they were notified about possible adverse reactions that might occur and the importance of monitoring their onset with the purpose of notifying them and adopting suitable measures to monitor them. Aside from verbal actions they were issued infographic material available on the websites of the Spanish Ministry of Health and General Council of the Official College of Pharmacists along the same lines, which reinforced the verbal information given.
Vaccinated patients with a reaction considered mild after its evaluation, were recommended the appropriate non-pharmacological measures and if necessary, a medicine was indicated without a suitable prescription to resolve or relieve the health problem caused by the reaction (figure 1).
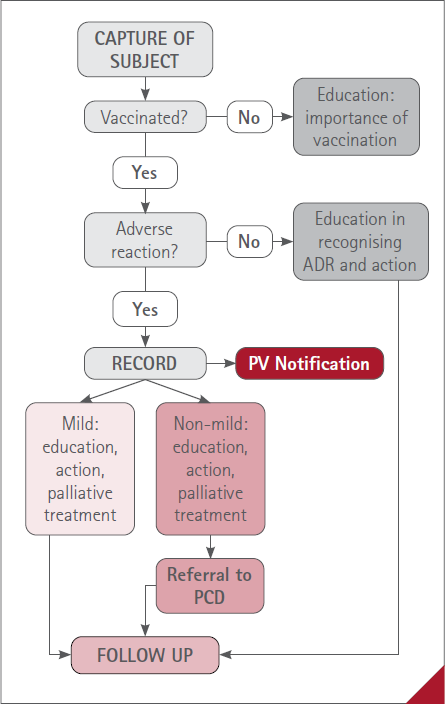
Figure 1 Summary of the procedure
Follow up of those vaccinated
As indicated in the procedure, all patients vaccinated were subject to active telephone or in person follow up (according to patient preference) by the collaborating pharmacist. (figure 2).
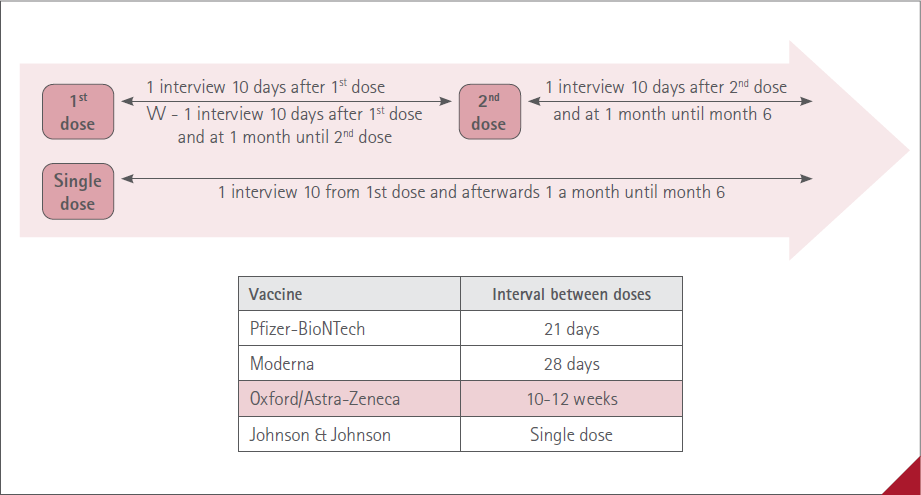
Figure 2 Summary of the follow up
Patients captured after the first dose
In the event of patients who only received the first dose or who received a single dose vaccine they underwent an initial phone/in person interview followed up 10 days after vaccination as long as this coincided before administration of the second vaccine dose. In the case of the Astra-Zeneca vaccine the monthly corresponding interviews were also held up until application of the second dose. Afterwards a monthly visit until month 6.
Patients captured after they received the second dose and patients in follow up from the first dose
First in person or phone interview 10 days from administration of the second dose. At the interview the data corresponding to the first dose were filled in. Subsequently, one a month until month 6. In the event it was deemed appropriate, the CP extended follow up for as long as necessary to verify course, possible onset of new suspected ADR and, as appropriate, the outcomes of any referral to the doctor.
Training of collaborating community pharmacists
The CP that took part in the activity received training by the research group in collaboration with the two provincial colleges by means of their online platforms, in general on pharmacovigilance, notification of ADR and COVID-19, and specific on the project’s methodology, the vaccines at issue in this work, their characteristics, action, reactions found in the clinical trials and specifications, procedure to follow given the onset of possible reactions, their evaluation and corresponding actions.
Presentation of results and statistical analysis
The statistical programme SPSS® 22.0 para Windows® was used for data analysis. Qualitative and qualitative data are shown as percentages and mean and standard deviation (SD), respectively. Chi-squared test was used to analyze qualitative variables, student t test for quantitative variables with a normal distribution and Mann-Whitney test for quantitative variables with a non- normal distribution. The Wilcoxon test was used for paired data analysis. To relate the quantitative variables Pearson or Spearman analytical correlation techniques were used. Statistical significance was set at P<0.05.
Ethics considerations
The study was developed in accordance with rules of Good Clinical Practice of the International Conference on Harmonization (ICH E6) for a study of these characteristics. There was compliance in all cases with the autonomy of subjects, according to the prevailing ethics principles of the Declaration of Helsinki.
At the time of the proposed incorporation into the study, the subject was issued with an information sheet and asked to sign an informed consent form.
The record sheets remained stored in collaborating pharmacies and complied with high level safety measures, as set out in Spanish Organic Law 3/2018, of 5 December, on the protection of personal data and guarantee of digital rights. The recorded data were subject to a coding and dissociation process (pseudonymisation), and loaded onto an Excel® spreadsheet before being notified to the research team.
The research team never had any knowledge of subjects’ identifying or identifiable data.
The study received a favourable opinion from the Galicia Research Ethics Committee for Medicines (RECm-G) (Exp. 2021-007) (Appendix 2).
RESULTS
Demographic data
A total of 10 pharmacies from the province of Pontevedra and two from Ourense collaborated. They proposed that 827 users take part, of whom 784 agreed (94.8%). Three were rejected as they had not recorded the data correctly, whereby the number of valid records was 781 (99.6%), of which 488 (62.5%) were women and 293 (37.5%) men. Mean age was 56.8 (SD=17.9) years. A total of eight (1.6%) women were pregnant.
Diseases reported: 230 hypertension, 177 dyslipidaemia, 159 neuro-psychiatric disorders, 105 cardiological, 92 diabetes, 68 respiratory, 50 thyroid, 35 obesity, 23 immunodepression, 17 renal, nine oncological, eight liver, six metabolic. Multipathological patients: two (0.3%) with eight pathologies, two (0.3%) with seven, 11 (1.4%) with six, 22 (2.8%) with five, 77 (9.9%) with four, 94 (12.0%) with three and 112 (14.3%) with two. With one pathology 216 (27.7%) and 245 (31.4%) manifested not suffering from any prior pathology. A total of 25 (3.2%) suffered from an acute pathology at the time of receiving the vaccine. The most common were: cold (4), vertigo (4), urine infection (2), anaemia (2) and labial herpes (2). Virtually half those surveyed were 60 or over and belonged to an at risk group. Only 64 reported having had COVID-19.
The demographic characteristics are shown in Table 1.
Table 1 Demographic characteristics by sex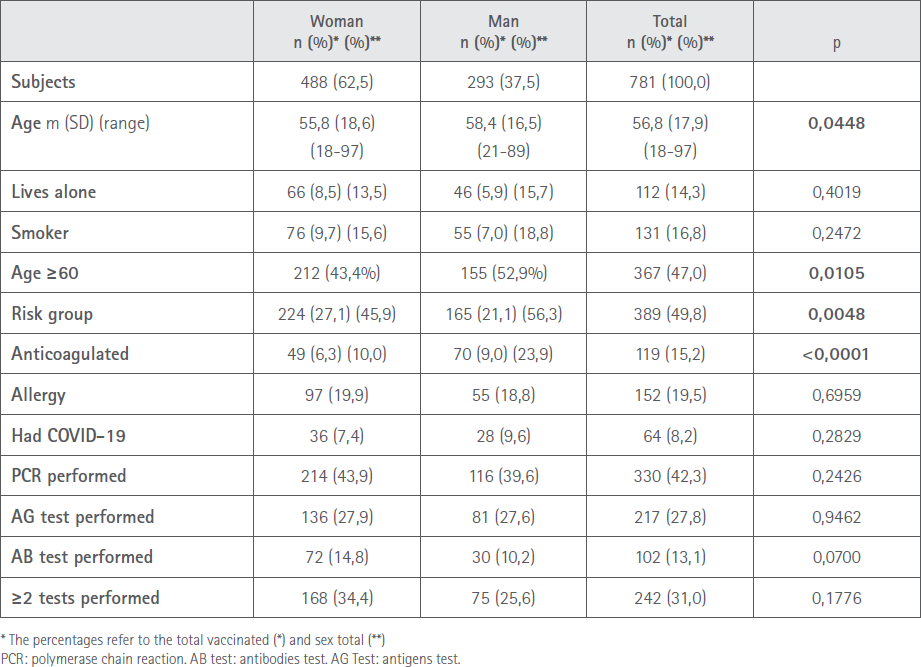
Vaccines
Vaccines were administered in massive vaccination areas (“vaccinodromes”) to 491 (62.9%), in a hospital 175 (22.4%), primary care centre 108 (13.8%) and old peoples home seven (0.9%).
A total of 445 (57.0%) subjects were vaccinated as a first dose with Comirnaty®, 282 (57.8%) women and 163 (55.6%) men; 190 (24.3%) received Vaxzevria®, 118 (24.2%) women and 72 (24.6%) men; 104 (13.3%) Spikevax®, 67 (13.7%) women and 37 (12.6%) men and 42 (5.4%) Jcovden®, 21 (4.3%) women and 21 (7.2%) men.
A total of 204 (26.1%) surveyed used medicines as prophylaxis for the possible ADR from the vaccine. Of these 165 (80.1%) took acetaminophen.
Suspected adverse reactions
A total of 495 (63.4%) vaccinated, 321 women (65.8%) and 174 (59.4%) men manifested having suffered at least one adverse reaction, P=0.0753. Tabla 2 shows the distribution of people who underwent ADR with the four vaccines according to sex.
The average number of ADR manifested by vaccinated people was 1.8 (SD=2.2) (1-13), 2.0 (SD=2.4) (1-13) in women and 1.4 (SD=1.7) (1-8) in men, P<0.0001. The percentage vaccinated in regard to the number of ADR reported by each person is shown in Figure 3. No statistically significant difference between sexes was detected (P=0.3164). The maximum was 13 ADR, in one woman.
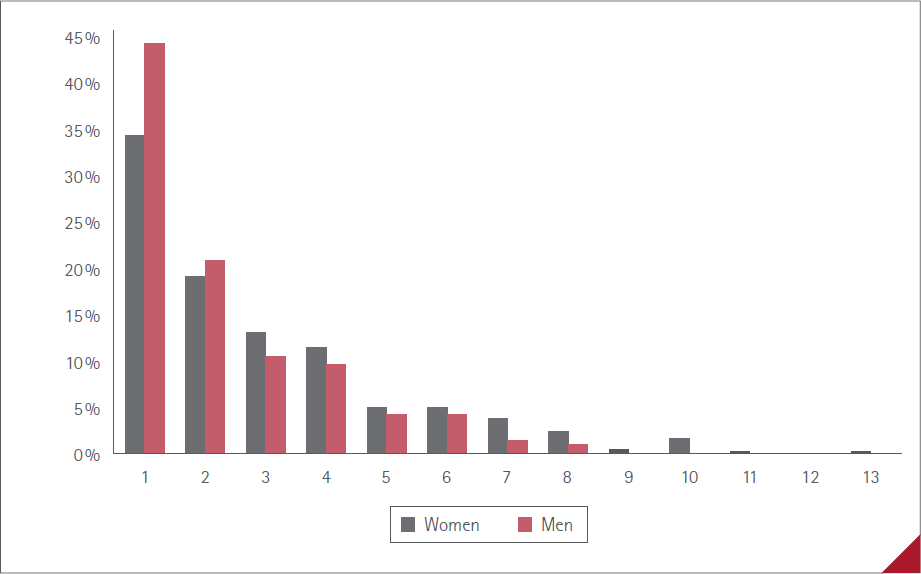
Figure 3 Percentage vaccinated and number of ADR manifested
The total number of ADR manifested by those surveyed was 1367. The most prevalent turned out to be those that affected more than 10% of those vaccinated: pain at the injection site, reported by 375 (48.0%), tiredness/fatigue, by 170 (21.8%), chills 118 (15.1%), headache 117 (15.0%), muscular pain 112 (14.3%) and fever 98 (12.5%).
The full list of suspected adverse effects manifested with the first dose is shown in Tabla 3.
Table 2 Distribution by sex and brand of vaccine of the subjects with ADR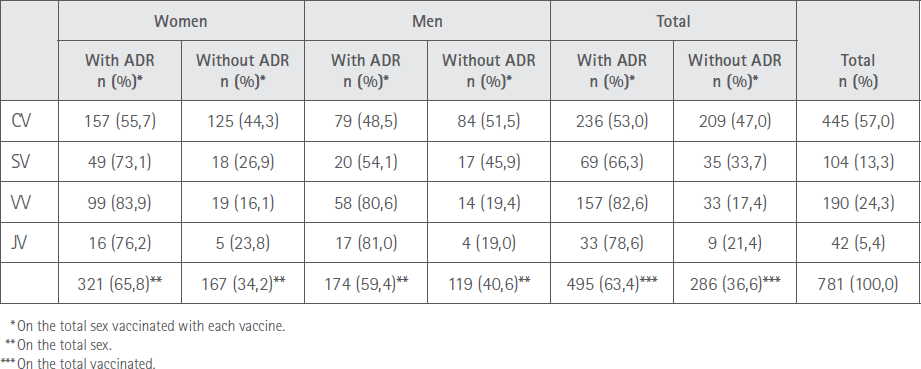
Table 3 Suspected ADR to the first dose manifested by subjects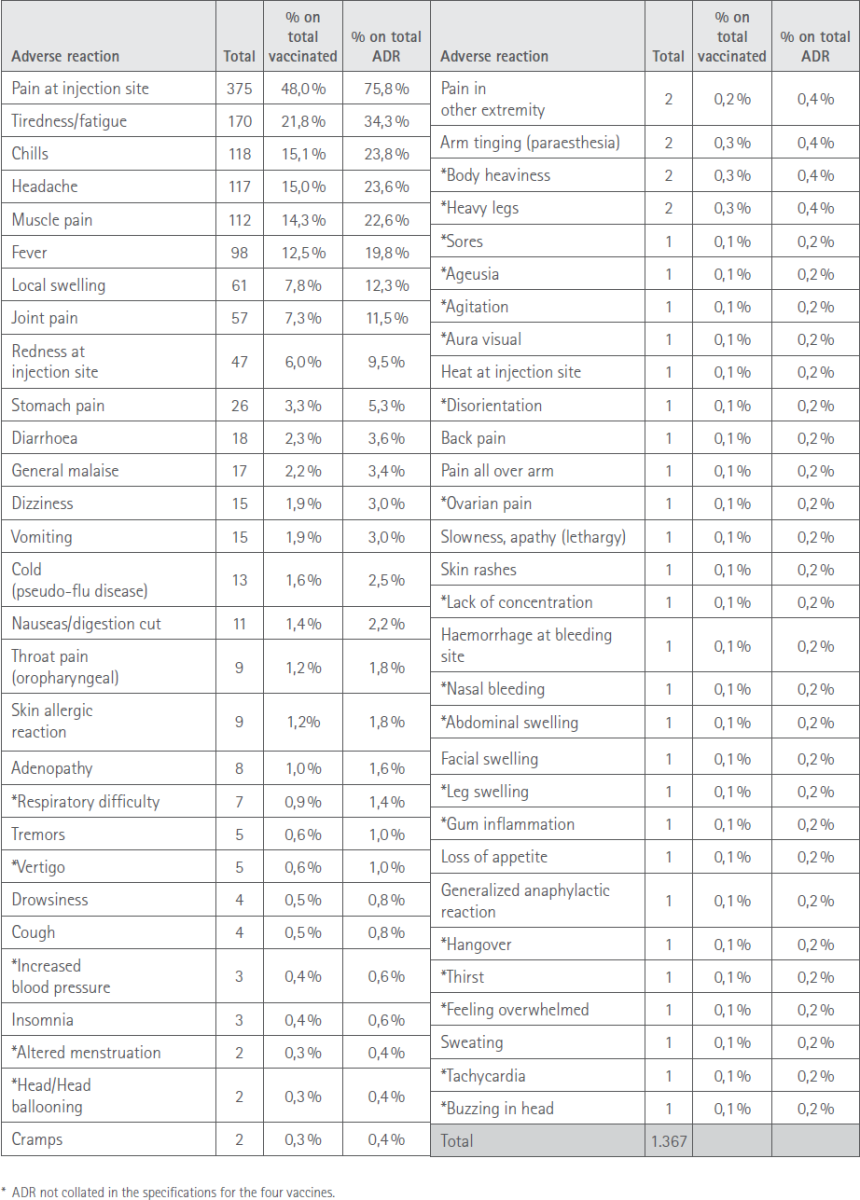
Table 4 Shows the percentage vaccinated of each sex that reported the most prevalent ADR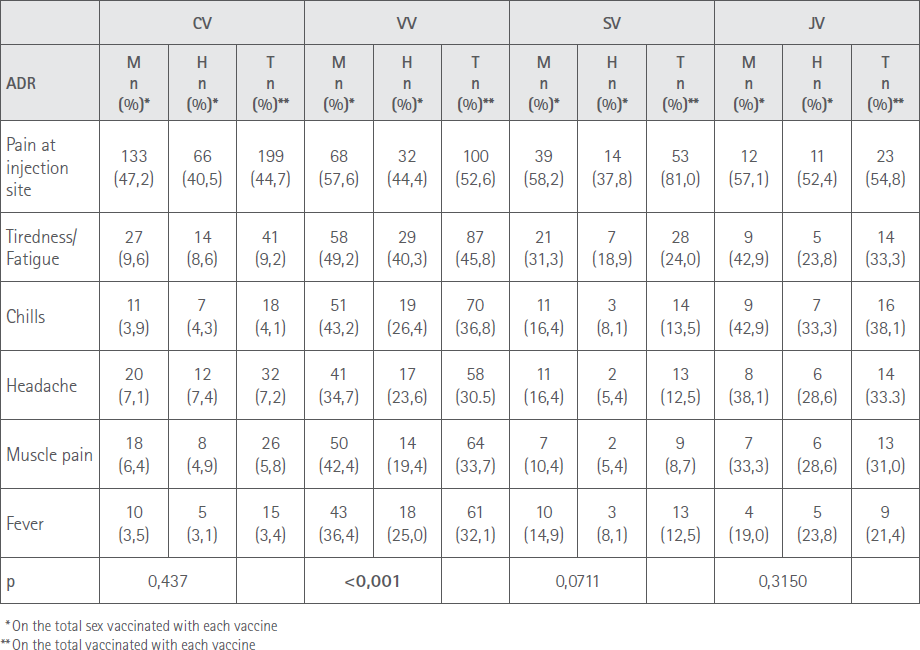
Table 4 shows the percentage vaccinated of each sex that reported the most prevalent ADR.
The analysis of relationships between the demographic characteristics of the sample and number of ADR manifested by subjects is shown in Table 5.
Table 5 Relationship between the number of ADR and demographic variables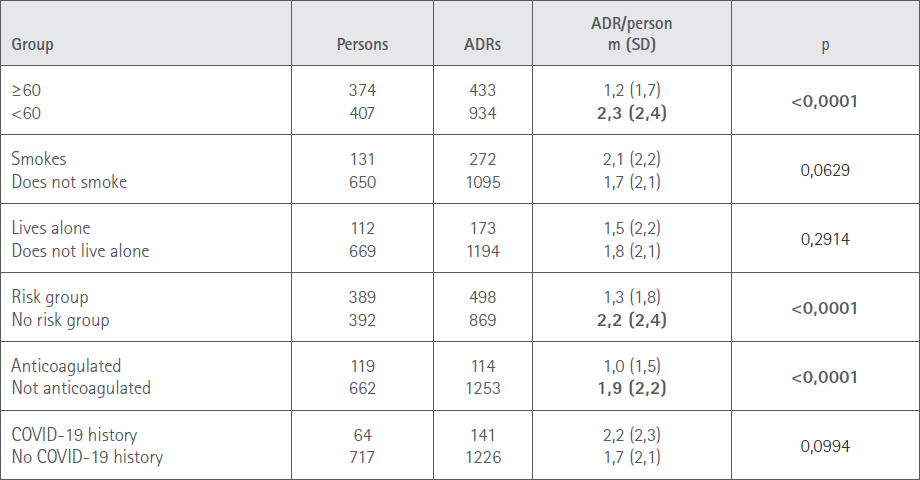
The average duration of ADR (time from onset to resolution) was under one day in 114 (8.3%) cases, one to three days in 999 (73.1%), four to five days in 141 (10.3%) and six days or more in 113 (8.3%). No statistically significant difference between men and women, P=0.4501. The duration of the ADR by sex is shown in Figure 4.
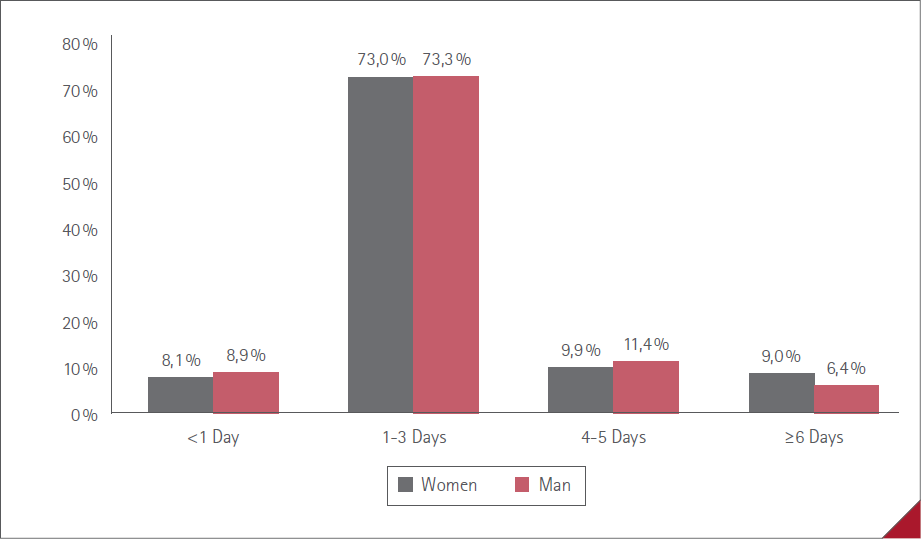
Figure 4 Duration of ADR by sex
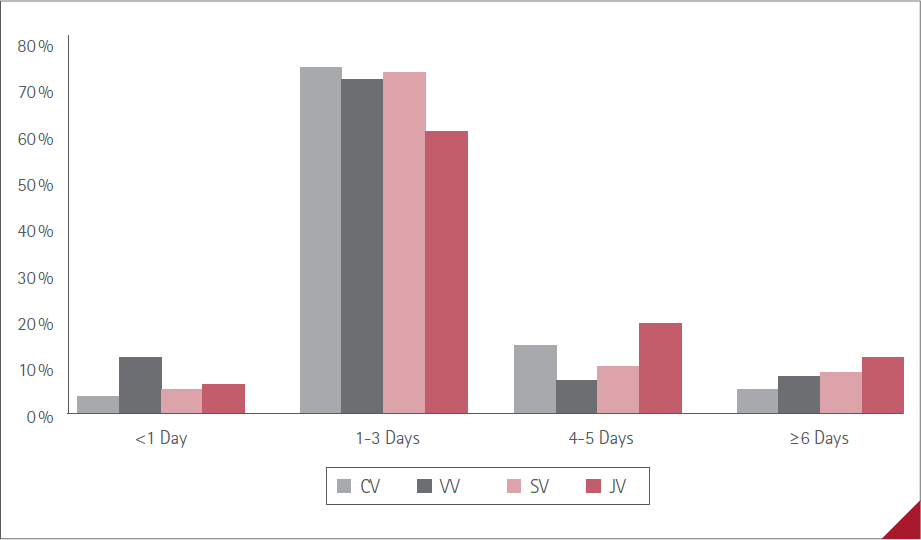
Figure 5 Duration of ADR with the four vaccines
The duration of ADR for each of the four vaccines administered is shown in Figure 5.
Impact on health and daily life
In 472 (95.4%) cases the health problem caused by the ADR resolved itself satisfactorily. Nonetheless, for 118 (15.1%) vaccinated, 76 (23.7%) women and 42 (24.1%) men (P=0.9087), the reactivity prevented them from performing their routine daily activity. After six months, 100% of cases had resolved.
Of the 495 people surveyed who manifested reactivity to the vaccine a total of 77 (15.6%) required professional help, of whom 48 (15.0%) were women and 29 (16.6%) men (P=0,6212): they visited their primary care doctor (PCD) at the health centre in 30 (39.0%) cases, (20 women and 10 men), nine (11.7%) (seven women and two men) at the casualty department, in addition to one by PCD referral and another from the CP, one (1.3%) man in hospital and 37 (48.1%) (21 women and 16 men) requested assistance in the pharmacy.
Collaborating pharmacists notified the Autonomous Pharmacovigilance Centre of the ADR from 264 (53.3%) people vaccinated.
DISCUSSION
Among the duties performed by the community pharmacist is monitoring the personalized use of medicines. The purpose is to detect the adverse reactions that might occur in the user population and notify them to the institutions responsible for pharmacovigilance [8-10].
According to this premise, and given that the main aim of this work is detection, notification and follow up of adverse reactions in community pharmacy users, it is worth noting that the outcomes obtained reveal that in its administration to the general population, the first dose of vaccines for COVID-19 led to a significant number of suspected ADR, although mild to moderate.
Study limitations
We wish to point out as a possible study limitation the fact that the manifestation of suspected ADR by participants has a certain component of subjectivity. In some cases, they could be attributed to pathologies or treatments that patients received, impacted state of mood, a family member or close friend having suffered the disease. All this may lead to a predisposition to recognize some that are actually not suspected ADR. However, the adverse reactions recorded in the pharmacy are usually mild to moderate in intensity. Those that are most severe are recorded in the hospital or emergency services, such as cases of thrombocytopenia, Guillain Barré syndrome or myocarditis. Many of these mild ADR do not usually reach these departments or are not recorded in them, whereby it is possible that the data differ in studies performed in them.
Furthermore, the COVID-19 vaccines themselves might cause the nocebo effect and alter their safety outcome. The at times negative advertising that accompanies the emergency authorization together with the lack of knowledge of adverse reactions and anticipation or fear of suffering from them increases the erroneous attribution of prior or subsequent symptoms actually unrelated to the vaccine [24].
Other limitations were the lack of access to the clinical history of the person surveyed to confirm the suspected ADR, characterize when it belongs to a risk group and confirm that COVID was diagnosed previously. The data depend on that manifested by subjects and the medicines they say they take or appear in the pharmacotherapeutic histories stored in pharmacies
Description of the sample
A total of 781 users took part with a mean age of 57; 62.5% were women, who mainly live alone (8.5% vs. 5.9% of the total sample). This datum coincides with that published by the Spanish Office of National Statistics for Galicia [25], doubtless due to the higher life expectancy of women compared to men [26]. Nonetheless, within each sex, in our sample the percentage is higher among men (15.7% vs. 13.5%).
As for tobacco consumption, our sample shows a higher percentage of male smokers, which also corresponds to the national statistic [27].
The number of those surveyed belonging to at risk [28,29] and anticoagulated groups is also higher among men. This latter case coincides with some studies, but not others as studies differ markedly in terms of anticoagulation and sex [30].
Consequently, we believe the sample to be sufficient, as shown by the calculation performed for the study’s representativeness. However, its composition matches the mainly female population that visits pharmacies, obtained in other studies [31,32,33].
Only 8.2% of those vaccinated state having gone through COVID-19 (36 women and 28 men) prior to receiving the vaccine. This datum differs from that published by Álvarez et al. [34] in which 50% of the study population had gone through the disease, whereby this can be accounted for by the different scope in which this study was performed.
Despite general prophylaxis with analgesics or anti-inflammatories not being recommended as we did not have clear data on the impact of these on the immune response [16,35], we found that a quarter of patients surveyed use a medicine for this purpose. Acetaminophen was the most commonly used (80.1%), doubtless due to lack of knowledge by the population of the vaccine’s possible adverse reactions and its fear of these.
The COVID-19 pandemic has led to significant challenges for the Spanish health system.
It needed to rearrange its services and transfer personnel to areas of most need. This was a viable solution, to respond suitably to the urgent problem of limited human resources, to undertake a massive and efficient vaccination of the entire population. We therefore found four different sites in our study: “vaccinodromes” (62.9%), hospitals (22.4%), primary care (13.8%) and old people’s homes (0.9%). The complexity to perform an effective vaccination and the need to equip primary care suitably to comply with the requirements set out meant that this was not a majority vaccination process. The success of vaccinodromes and decentralized areas as compared to hospitals, the latter reserved to treat the most severe cases, was clear. The study of how to implement this model of more decentralized and accessible areas, among which may be pharmacies, as occurs in many countries in our setting, to treat or prevent certain health problems and decongest the health services would be suitable [36].
Those surveyed in our study received four different kinds of vaccine: Cominarty® and Spykevax®, messenger RNA vaccines, Vaxzervia® and Janssen® both with adenovirus as a vector. The proportions of vaccines received by subjects in our study do not fully coincide with the data shown in the Spanish Ministry of Health’s reports, as these account for vaccines administered to date, whilst ours are limited to the year 2021. Cominarty® does coincide as the most commonly used brand [37].
Suspected adverse reactions
Over 60% of subjects in the study manifested at least one ADR. This figure is high and does not concur with those collated in other reports, which varies from 0.08% in Galicia [38] to 0.18% nationally [39] in which data are collated from notifications to the Spanish pharmacovigilance system, most coming from medical and nursing consultations which probably only reflect moderate to severe ADR that fall within this scope. In a tertiary Madrid hospital [34] this was 2.5% with the first dose and this increased to 4.8% after the second dose. This also involved notifications, in this case to the Department of Prevention of Work-Related risks. In two studies performed with health professionals with voluntary response surveys, in the event of nephrologists, 75% of those who responded had undergone ADR to the vaccine [40]. Among community pharmacists from Pontevedra, this figure was 92% [41]. Although the figures are skewed by the methodology, we believe they highlight an actual prevalence of ADR much higher than that collated in specifications and official reports, as suggested by Demasi [42].
The total number of suspected ADR with the first dose of vaccines was 1367. The most prevalent was pain at the injection site, which affected 48% of those vaccinated and entailed 75.8% of ADR notified. This coincides with other studies published [17,24,40,43], followed by tiredness, fatigue and chills, especially with VV and JV, which appeared in the 11th PV report of the Spanish Ministry of Health [44]. The onset of headache and muscular pain except for chills, in the same order as the study by Amanzio et al [24]. In the others, despite coinciding with the most prevalent ADR, the order differs in all of them [34,38-40,43].
On balance, we observe that in our study the most commonly administered vaccine was CV (57.0%), which is the one that led to the fewest ADR (53,0%). VV was the next most administered (24.3%) and the one that led to the most ADR (82.6%). This appears to indicate that the mRNA vaccines were the safest.
Various suspected ADR not covered in the specifications of the four vaccines administered were detected. While as we mentioned we cannot ensure a proven causal relationship with the vaccine, we believe that some are quite relevant, among them the respiratory difficulty and vertigo or menstrual abnormalities and ovary pain. However, they still do not appear in the corresponding specifications [20-23]. We also found a case of tachycardia with Comirnaty®, in a 72-year-old woman without a history of having suffered from the disease, with onset at six hours and duration one day. This ADR, which was not covered in the specifications either, was reported by Marco et al [45], in three health professionals who had undergone the disease, with remission between 18 and 36 hours.
The percentage of women who manifested ADR (66%) is greater than the percentage of men (59%), which coincides with that found in the reports cited [34,38,39], although the difference is not statistically significant. The number of adverse reactions notified per person is 1.8 in the total sample, in this case with a statistically significant difference between men (1.4) and women (2.0). When studying the number of adverse reactions in regard to the other demographic variables, we detected that those under 60 had a higher number of ADR per person (2,3) compared to those 60 or over (1,2), which coincides with that reported in the bibliography [39,43] and specifications [20-23]. There is also a significant difference between those vaccinated in at risk and anticoagulated groups and those not belonging to these groups. This is a relationship not analyzed in other studies, whereby we cannot set out comparisons but we can interpret as associated the higher age of those included in these groups.
No statistically significant differences in regard to being smokers, living alone or being infected with COVID-19 prior to vaccination were detected. In this latter case, this fact is considered a predictor of the onset of symptoms after vaccination in other studies [34,40,43,45].
As these are mild ADR in general, their duration was short. This was prolonged more than 5 days only in 8% of cases, between 1 and 3 days for 73% of those surveyed. This datum coincides with other published studies that reported a duration of symptoms 1-4 days, more in women than men [43,46], which did not occur in our study in which the duration of symptoms was similar in both sexes.
Impact on health and daily life
Aside from this fast recovery, in 95% of cases, reactions resolved spontaneously. Only in under 5% of cases did symptoms remain for more than a month, but at the closing of data recording for the study all of them had been resolved. Nonetheless, for 15% of those surveyed the discomfort caused by reactivity to the vaccines prevented them from performing their normal activity, either work or daily life routines. This is an important aspect for people vaccinated both for active workers and the retired, which was not considered in the reports we have reviewed and that might have a certain impact on the subsequent perception on the hazardousness of vaccines.
A total of 16% of those vaccinated who underwent ADR required professional help to resolve the health problem caused by these. Therefore, approximately half came to the pharmacy requesting the CP’s attention, ratifying the trust of the population in her proximity, accessibility and capacity at times of saturation of public healthcare services. The CP evaluated whether or not the ADR was severe, referring the physical or emergency department if deemed necessary or indicating a medicine and/or pharmacological measures to relieve symptoms. This work, performed by the CP not only in our study but in general for vaccinated persons who visited pharmacies manifesting symptoms compatible with reactivity to COVID-19 vaccines, meant a significant contribution to the health system to collaborate on rationalization of the use of public health resources, with considerable cost savings and avoiding unnecessary visits to health and emergency centres, and acting as an efficient first primary health care step.
We hope to have contributed with this work, which will continue with the analysis of the ADR detected after the second dose of vaccines and comparison between both doses, to better knowledge of their safety profile having highlighted various suspected ADR, some quite relevant, which were not collated in the specifications.
Moreover, as discussed and with the limitations mentioned, we believe that the reports from the Spanish health authorities do not cover all the ADR that occurred because of COVID-19 vaccines, or the actual number of people vaccinated who underwent ADR after vaccination. Possibly this is a problem of under notification to the Spanish pharmacovigilance system of mild ADR, both by health professionals and those vaccinated, which in our case was collated. Nor do they reflect the impact on the daily, work and personal life of those vaccinated, which from our point of view has been considerable; without this undermining the transcendental benefit obtained with its administration, both on the loss of lives and reduction of suffering and sequelae caused by the severe cases, which have fallen drastically and have enabled a return to relative normality after almost two years of pandemic.
We believe this kind of work will serve to strengthen the key position of the community pharmacist in Spanish primary health care structures and sought after community mechanisms and collaboration with other health professionals. Taking part from a community position, which is close and accessible to users, despite the pandemic, at a time when health centres and hospitals avoided personal care of patients with the purpose of minimizing health risks.
ACKNOWLEDGEMENTS
To the 784 vaccinated persons who agreed to join our project and take part in subsequent follow up. It would not have been possible to carry this out without the availability, patients and dedication of all of them.
Collaborating pharmacists: Farmacia Carballido (Ourense): Rosa Mª Carballido Gago, Mª Pilar González Abades; Farmacia MT Rodríguez (Ourense): Mª Teresa Rodríguez Rodríguez, Laura León Rodríguez, Ruben Alonso Bailez, Lara Fernández Puga; Farmacia Rey Carballeda (Pontevedra): Belén Rey Carballeda, Begoña Gago Arca; Farmacia Andrés (Vigo): J. Carlos Andrés Iglesias, Rocío Mera Gallego, Alex Piñeiro Abad; Farmacia Colmenero (Vigo): Patricia López Colmenero, Miriam Barreiro Juncal, Laura Pérez Molina; Farmacia Acea (Vigo): Raquel Acea Lorenzo, Mónica González Blanco; Farmacia Mallada (Vigo): Álvaro Mallada Garabato, Diego López Cantorna; Farmacia Fornos (Cangas do Morrazo): José A. Fornos Pérez, Patricia García Rodríguez, Lorena Tenorio Salgueiro; Farmacia Cordeiro (Moaña): Marta Fernández Cordeiro; Farmacia Cebreiro (Tui): Mª José Cebreiro Mosquera, Carla Novás García; Farmacia Rodríguez, (Crecente): Ramón Rodríguez Álvarez, Bibiana Guisado Barral, Xurxo Diz Gerpe; Farmacia Alonso (O Rosal): Ricardo Alonso Pérez, Yésica Oza Araujo.
Our thanks also go to the staff of the Pontevedra and Ourense COP Technical Department for their support and dissemination of our project among its college members.
REFERENCES
1. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). [Consultado 22/8/2022]. Disponible en: https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6
2. Ministerio de Sanidad. Actualización nº 626. Enfermedad por SARS-CoV-2 (COVID-19). 19/8/2022. [Consultado 22/8/2022]. Disponible en: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_626_COVID-19.pdf
3. Ministerio de Sanidad. La EMA recomienda la autorización de la primera vacuna frente a la COVID-19 [Nota informativa]. 21/12/2020. [Consultado 25/7/2022]. Disponible en: https://www.aemps.gob.es/informa/notasInformativas/laAEMPS/2020/docs/NI-AEMPS-38-2020- vacuna-HMA.pdf?x54046
4. European Commission. EU Vaccines Strategy. Authorised Vaccines 2021. [Consultado 25/7/2022]. Disponible en: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/public-health/eu-vaccines-strategy_en#authorised-vaccines
5. Ministerio de Sanidad. La AEMPS lanza una campaña sobre las garantías de las vacunas frente a la COVID-19. [Nota informativa]. 21/12/2020. [Consultado 27/7/2022]. Disponible en: https://www.aemps.gob.es/informa/notasinformativas/laaemps/2020-laaemps/la-aemps-lanza-una-campana-sobre-las-garantias-de-las-vacunas-frente-a-la-covid-19/
6. Ministerio de Sanidad. Vigilancia de la Seguridad de las vacunas frente a la COVID-19 V-6. 17/12/2020. [Consultado 27/7/2022]. Disponible en: https://www.aemps.gob.es/medicamentosUsoHumano/vacunas/docs/vigilancia_seguridad_vacunas_COVID-19.pdf?x54046&x95597
7. Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. BMJ. 1998;316(7140):1295-8. doi:10.1136/bmj.316.7140.1295
8. Ley 33/2011 de 4 de octubre, General de Salud Pública. Boletín Oficial del Estado, Núm. 240 de 5 de octubre de 2011. Disponible en: https://www.boe.es/eli/es/l/2011/10/04/33
9. Real Decreto 577/2013, de 26 de julio, por el que se regula la farmacovigilancia de medicamentos de uso humano. Ministerio de Sanidad, Servicios Sociales e Igualdad. BOE nº 179, de 27 de julio de 2013. Disponible en: https://www.boe.es/eli/es/rd/2013/07/26/577
10. Ley 3/2019, de 2 de julio, de ordenación farmacéutica de Galicia. Diario Oficial de Galicia, nº 130 de 10 de julio de 2019. Págs. 32380- 32447. Disponible en: https://www.boe.es/buscar/pdf/2019/BOE-A-2019-13517-consolidado.pdf
11. Lorenzo Veiga B, Marcos González L, Acuña Ferradanes A, Mera Gallego R, Vérez Cotelo N, Andrés Iglesias JC, et al. Farmacovigilancia en farmacia comunitaria de medicamentos recientemente comercializados. Pharm Care Esp. 2015;17(3):360-75. Disponible en: https://www.pharmcareesp.com/index.php/PharmaCARE/article/view/235
12. Alonso Lovera P. Farmacovigilancia de psicofármacos en una farmacia de A Coruña (España). Farmacéuticos Comunitarios. 2016;8(1):5-12. doi:10.5672/FC.2173-9218.(2016/Vol8).001.02
13. Acta Sanitaria. El COFM reconoce la labor de los integrantes de la Red de Farmacias Centinela de Madrid. Actualizado: 2018. [Consultado 23/7/2022]. Disponible en: https://www.actasanitaria.com/cofm-reconoce-integrantes-red-farmacias-centinela/
14. Acta Sanitaria. Farmacias centinela de Castilla y León detectan más de un millar de problemas con fármacos. Actualizado: 2020. [Consultado 23/7/2022]. Disponible en: https://www.actasanitaria.com/farmacias-centinela-de-castilla-y-leon-detectan-mas-de-un-millar-de-problemas-con-farmacos/
15. Acta Sanitaria. Castilla-La Mancha pone en valor el papel de la Red de Farmacias Centinela. Actualizado: 2019. [Consultado 23/7/2022]. Disponible en: https://www.actasanitaria.com/castilla-la-mancha-pone-valor-papel-la-red-farmacias-centinela/
16. Xunta de Galicia. Dirección Xeral de Saúde Pública. Plan galego de vacinación fronte ao SARS-CoV-2. V8.2 7/4/2022. [Consultado 10/8/2022]. Disponible en: https://coronavirus.sergas.gal/Contidos/Plan-galego-vacinacion-COVID
17. Polack F, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2020. doi:10.1056/NEJMoa2034577
18. Anderson EJ. Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383:2427-38. doi:10.1056/NEJMoa2028436
19. Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020. Disponible en: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext
20. Agencia Europea del Medicamento. Ficha técnica Comirnaty®. [Consultado 16/8/22]. Disponible en: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_es.pdf
21. Agencia Europea del Medicamento. Ficha técnica Spikevax®. [Consultado 24/7/22]. Disponible en: https://www.ema.europa.eu/en/
22. Agencia Europea del Medicamento. Ficha técnica Vaxzevria®. [Consultado 24/7/22]. Disponible en: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_es.pdf
23. Agencia Europea del Medicamento. Ficha técnica COVID-19 Vaccine Janssen®. [Consultado 24/7/22]. Disponible en: https://ec.europa.eu/health/documents/community-register/2021/20210311151284/anx_151284_es.pdf
24. Amanzio M, Mitsikostas DD, Giovannelli F, Cipriani GE, Brown WA. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: A systematic review, The Lancet Regional Health. 2022 Ene; 5(1):e2143955. doi:10.1016/j.lanepe.2021.100253
25. Instituto Nacional de Estadística. Número de hogares unipersonales por comunidades y ciudades autónomas según sexo, edad y estado civil. 2020. [Consultado 15/7/2022]. Disponible en: https://www.ine.es/jaxi/Tabla.htm?path=/t20/p274/serie/prov/p02/l0/&file=02014.px&L=0
26. Instituto Nacional de Estadística. Salud: esperanza de vida. 2022. [Consultado 15/7/2022]. Disponible en: https://www.ine.es/ss/Satellite?L=es_ES&c=INESeccion_C&cid=1259926380048&p=1254735110672&pagename=ProductosYServicios/PYSLayout
27. Instituto Nacional de Estadística. Salud: consumo de tabaco según grupo de edad y periodo. 2022. [Consultado 15/7/2022]. Disponible en: https://www.ine.es/jaxi/Datos.htm?path=/t00/mujeres_hombres/ tablas_1/l0/&file=d07001.px
28. Ministerio de Sanidad. Información Científica-Técnica. Enfermedad por coronavirus, COVID-19. Actualizado2/6/2020. [Consultado 13/6/2020]. Disponible en: https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/ITCoronavirus.pdf
29. Ministerio de Sanidad. Procedimiento de actuación para los servicios de prevención de riesgos laborales frente a la exposición al SARS-CoV-2 8/6/2020. [Consultado 13/6/2020]. Disponible en: https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/PrevencionRRLL_COVID-19.pdf
30. Jorge Araujo P. El perfil del paciente anticoagulado de un centro de salud de Gran Canaria. 02/04/2019. [Consultado 17/8/2022]. Disponible en: https://revistamedica.com/paciente-anticoagulado/
31. Giménez M, Giménez MP, Giménez M, Muñoz MF, García J. Perfil de pacientes y hábitos de consumo en una farmacia comunitaria, 150 años después. Farm Comunitarios. 2016 May 26;8(Supl. 1). Disponible en: https:// www.farmaceuticoscomunitarios.org/es/journal-article/perfil-pacientes-habitos-consumo-una-farmacia-comunitaria-150-anos-despues
32. Zalve JL, Gómez A, Martínez R, Pardo E, Córdoba A, Garrido E. Proyecto ‘RUMBO. Estudio de la experiencia de paciente en la farmacia comunitaria española’: perfil de los pacientes que acuden a las farmacias comunitarias en España. Farm Comunitarios. 2018;10(Supl. 1):334. Disponible en: https://www.farmaceuticoscomunitarios.org/es/journal-article/proyecto-rumbo-estudio-experiencia-paciente-farmacia-comunitaria-espanola-perfil
33. Mera-Gallego R, León-Rodríguez L, Mera-Gallego I, González-Blanco M, Fernández-Cordeiro M, Piñeiro-Abad A, et al. Percepción de los usuarios de la farmacia comunitaria sobre la COVID-19 al final de la alarma y comparación con la situación al inicio. Farm Comunitarios. 2021 Jan 20;13(1):7-16. doi:10.33620/FC.2173-9218.(2021/Vol13).001.03
34. Álvarez L, Castiñeiras M, González F, Gonzáles JM, Casma RM, Nuñez MC. Reacciones adversas notificadas tras la administración de vacuna frente a COVID-19 en trabajadores de un hospital terciario. Rev Asoc Esp Med Trab. 2021; 30(2): 125-261. Disponible en: https://scielo.isciii.es/scielo.php?script=sci_abstract&pid=S1132-62552021000200217&lng=es
35. Lobos CG. Uso del paracetamol como profiláctico o postvacunación: revisión de la evidencia. [Internet] Farmacovigilancia de vacunas. 2020. [Consultado 15/8/2022]. Disponible en: https://www.ispch.cl/newsfarmacovacunas/2020/08/03%20_Vigilancia_ESAVI_1.pdf
36. Ares-Blanco S, Astier-Peña MP, Gómez-Bravo R, Fernández-García M, Bueno-Ortiz JM. Gestión de los recursos humanos y estrategias de vacunación en atención primaria en Europa en la pandemia COVID-19. Aten Primaria. 2021; 53(10):102132. doi:10.1016/j.aprim.2021.102132
37. Ministerio de Sanidad. Gestión integral de la vacunación COVID-19. 10/8/2022. [Consultado 18/8/2022]. Disponible en: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Informe_GIV_comunicacion_20220812.pdf
38. Xunta de Galicia. Prevención COVID-19. Acontecimientos adversos notificados en Galicia. Boletín de Farmacovixilancia. 2022; 1. [Consultado 18/8/2022]. Disponible en: https://libraria.xunta.gal/es/boletin-de-farmacovixilancia-no-1-2022
39. Ministerio de Sanidad. 16º Informe de farmacovigilancia sobre Vacunas COVID-19. 20/7/2022. [Consultado 18/8/2022]. Disponible en: https://www.aemps.gob.es/informa/boletines-aemps/boletin-fv/ 2022-fv/16o-informe-de-farmacovigilancia-sobre-vacunascovid-19/
40. Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: Acceptance and side effects. Journal of Healthcare Quality Research.2021; 36(6):236-369. doi:10.1016/j.jhqr.2021.05.002
41. Andrés-Rodríguez NF, Fornos-Pérez JA, Busto-Domínguez I, Mera-Gallego R, García-Rodríguez P, Carrera-Pérez-de-Juan MD, León-Rodríguez L, Mera-Gallego I, Acuña-Ferradanes A. Efectos adversos de las vacunas frente al SARS-CoV-2 en farmacéuticos comunitarios de Pontevedra. Farm Comunitarios. 2022 Jul 21;14(3):15-21. doi: 10.33620/FC.2173-9218.(2022/Vol14).003.03
42. Demasi M. Are adverse events in COVID-19 vaccine trials under-reported? 23/11/ 2021. [Consultado 19/8/2022]. Disponible en: https://maryannedemasi.com/publications/f/are-adverse-events-in-covid-19-vaccine-trials-under-reported
43. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021 Jul; 21 (7):939-949. doi:10.1016/S1473-3099(21)00224-3
44. Ministerio de Sanidad. 11º Informe de farmacovigilancia sobre Vacunas COVID-19. 20/12/2021. [Consultado 17/8/2022]. Disponible en: https://www.aemps.gob.es/informa/11o-informe-de-farmacovigilancia-sobre-vacunas-covid-19/
45. Marco García MT, Torres Lana A, M. Anta Agudo MB, Rufino Delgado MT. Taquicardia como efecto adverso no descrito en la vacuna Comirnaty© (vacuna COVID-19 mRNA BNT162b2 de Pfizer-BioNTech): descripción de 3 casos con antecedentes de SARS-CoV-2. Enfermedades Infecc. y Microbiol. Clin. 2022;40(5):276-277. doi:10.1016/j.eimc.2021.03.008
46. Al Khames QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahha, AT, et al. Safety of COVID-19 vaccines. J Med Virol. 2021;93 (12):6588-6594. doi:10.1002/jmv.27214
Editor: © SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria.
Copyright© SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria. This article is available from url https://www.farmaceuticoscomunitarios.org/. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en


