- The journal
- 👟 кроссовки adidas streetball cordura адидас / наложка bs👟 — цена 2540 грн в каталоге Кроссовки ✓ Купить мужские вещи по доступной цене на Шафе , adidas Originals Sustainable Stan Smiths Vita sneakers med heltäckande grafiskt mönster , Украина #126359384
- air jordan 1 low outlet
- Мужские россовки adidas decade hi b - Украина #28353944 , adidas Originals Vit t-shirt i boyfriend-modell med stor logga - ball оригинал кожа — цена 2900 грн в каталоге Кроссовки ✓ Купить мужские вещи по доступной цене на Шафе
- nike flyknit roshe electric green black Paris 308270 - SBD - nike boot with side zipper jeans black pants size , 111 Release Date
- Air Jordan Release Dates 2023 , Drip Bar Detroit , AIR Spizike JORDAN
- adidas y 3 qasa high triple black
- air jordan release dates
- kids air jordan
- Gnarhunters Nike SB Dunk Low DH7756 010 Release Date On Foot
- adidas Ultra Boost 2022 COLD.RDY Magic Grey GZ0128 Release Date
- Aims & Scope
- Editorial Policies and Processes
- Scientific and Methodological Rigor
- Production and Administration
- Committees
- Authors
- Ethical Considerations
- Submit Article
- Archive
- Indexation
- Search
- Contact us
Farm Comunitarios. 2023 Apr 14;15(2):5-11. doi: 10.33620/FC.2173-9218.(2023).10
Prospective observational study of the impact of problems accessing treatment such as risk factors for adherence and persistence. Pilot study
INTRODUCTION
Adherence is defined as the patient’s actual degree of agreement with the guidelines prescribed by a health professional; whilst persistence is defined as the duration of time from when a treatment commences until it is discontinued (1). The World Health Organization (WHO) notifies that “the consequences of a low adherence in chronic treatments are low health outcomes and increased healthcare costs” (2). This is broadly verified in various studies and with obvious impact on patients’ lives (3-5). For its part, the risk factors associated with adherence and persistence are markedly studied (in regard to the patient, treatment, pathology, family and health system) (6).
However, in Spain there is less knowledge of risk factors related to the health system; specifically to problems accessing treatment arising from the system’s inefficiency that can turn into actual barriers and, therefore, increase the lack of adherence and/or persistence.
One of the first studies was performed in the province of Zaragoza on the impact generated by pharmaceutical replacement or size of container by the community pharmacist in the event of emergency or shortage. In this study a total of 15,093 replacements were included within exceptional dispensing over a two-year period (May 2014-April 2016), in which in addition to ensuring correct access to the drug and persistence of the treatment, savings of €547,121 were attributed to the Spanish Health System (Spanish NHS) for medical consultations avoided (7).
Another preliminary study was performed in Asturias (8) where new risk factors were identified in the face of maintained adherence and persistence associated with lack of access to drugs including problems with shortage (temporary or definitive), expired prescription, pharmaceutical form, size of the container, suitability of a determined presentation or the patient’s loss of the medication. The total case incidence rate stood at 1.26% of total dispensing.
The reality of the COVID-19 pandemic has made this problem even more visible (9). For example, forcing the system to extend the prescription’s expiry. Shortage is also a growing problem as shown by the recent report from the Spanish Agency for Medicines and Health Devices, AEMPS (10). For example on the date of writing this paper there is a problem with supply of amoxicillin causing severe problems in the paediatric population. Therefore, AEMPS has agreed to allow exceptional dispensing in this unique case with the aim of resolving this severe situation (11).
In short, the lack of access to the treatment because of the new causes set out is a modifiable risk factor, which impacts health outcomes. Therefore, a pilot study needs to be performed that enables properly evaluating the current problem attributable to the health system of the difficulty accessing pharmacological treatments.
AIMS
The main aim of this study is to estimate the incidence of health system related risk factors associated with problems accessing community pharmacy treatments, risk factors that can alter adherence and persistence.
The secondary aim is to estimate the potential variations of the incidence of health system related risk factors according to the subgroups defined in the study.
MATERIAL AND METHODS
The study design is observational, prospective, transversal and randomized by strata.
The scope of the study was community pharmacies in Asturias and Aragón. These were chosen because Asturias and Aragón are two autonomous communities with a high proportion of medicine users, mainly the affected population.
The study was performed from 8/11/2021 to 21/11/2021.
The target population was outpatients visiting pharmacies in Asturias o Aragón with an e-prescription or medical report issued by their respective autonomous health departments.
Prescriptions from a health system professional in autonomous communities, as paper e-prescription or medical report were included as inclusion criteria. They had to be quantifiable because of presenting a risk factor that hindered its dispensing. And as exclusion criteria prescriptions from a professional not belonging to the corresponding autonomous community health system or prescriptions made privately in any format.
The study unit was the community pharmacy. Two variables were considered for correct stratification of the sample and correct representation of the target population: type of pharmacy and province. Pharmacies were classified into three kinds according to size of the population they serve, rural (<5000 inhabitants), semi-urban (5000-50,000 inhabitants) and urban (>50,000 inhabitants).
The sample size, 143 pharmacies, was calculated based on an incidence similar to that of the initial study in Asturias (8). A maximum error of 3%, 95% confidence level and statistical power 80% was selected. The estimated sample increased by 50% in forecast of the expected losses.
The sample was selected randomly. Therefore, a list of pharmacies to contact was drawn up for each “Province-Type” strata assigning each pharmacy a number. A table of random numbers was generated without repetition. This determined the order in which the pharmacies from each group were called by phone until the quota of 143 pharmacies necessary to complete the sample was full; an 50% abandonment rate was considered.
The primary endpoint was health system related risk factors meaning the prescription adapted for the study cannot be dispensed (12)
Health system related risk factors
I. Problem with supply of the prescribed drug.
The legal replacement in Spain by the community pharmacist is restricted to a replacement under bioequivalence conditions and with the same pharmaceutical presentation, dose and form.
Ia. Replaceables
Those cases in which the prescribed drug or its possible substitutes are found to be in a recognized condition of shortage by the AEMPS or they have been withdrawn because of health notification. Other alternatives exist on the market that guarantee an identical bioavailability.
Ib. Non-replaceables
When non-replaceable medicines are at issue, these cannot be replaced by others with the same active substance without the intervention of the prescribing physician.
II. Expiry of the prescription
IIa. Initial dispensing
Treatments that have exceeded the days set out for their first dispensing and are deactivated.
IIb. Continuation treatments
The prescription has exceeded the prescription’s review time without the patient having undertaken any action to renew it; in addition to cases of treatment in which the patient has already withdrawn an initial treatment box with paper prescription but without activating the e-prescription because the medication is insufficient.
III. Prescription with absence/defect in the prescription
IIIa. Justified cause
Recordable loss of prescription or drug, breakage, container defect.
IIIb. Document invalid as a prescription for the Spanish health system
The document presented does not have the official prescription format as occurs with the hospital report or the prescription is incorrectly filled in or lacks a requirement such as the approval.
Note. The request for a drug by the patient without there being a prior prescription is ruled out as a possibility for exceptional dispensing and is not considered in the study.
IV. Prescription with inadequate Pharmaceutical Form (PF) for the patient
Possible risk factor for discontinuation of the treatment as the prescribed pharmaceutical form is not suitable for the patient according to her characteristics, the pharmacist’s professional criteria or the patient’s own opinion.
V. Insufficient dose prescribed to complete the treatment
Prescription of a number of doses lower than what is required to complete the treatment in the number of days set out by the doctor.
Secondary endpoints were classification of the pharmacy (urban, semi-urban or rural), patient’s sex and age, national code (NC) of the drug affected, number of drugs the patient has prescribed as e-prescription, duration of the treatment (chronic/acute), the patient’s type of associated contribution (Individual Health Card, IHC) and care level (primary/hospital care).
Data were collected by means of recording all dispensing that could not be performed. Once the risk factor was identified data were recorded in a case report form designed ad-hoc for the study (Appendix).
A training session was prepared on two different days. There was daily record by the pharmacists throughout the pharmacy’s opening hours and whilst the study lasted. The total dispensing that day was also recorded merely for statistical purposes.
Frequency measurements were calculated by means of the IBM SPSS Statistics v. 20.0 programme. Frequency of the primary endpoint (number of prescriptions subject to incidence) with subsequent breakdown of absolute and relative frequencies according to secondary endpoints (sex, age, type of pharmacy, IHC, care level and therapeutic groups at issue).
RESULTS
A total of 143 pharmacies were randomly assigned to the study, of which 98 began and completed the study.
In all 138,697 valid dispensing operations were performed and 2221 incidences were detected corresponding to 2009 patients. The basal characteristics of patients who presented an incidence are shown in Table 1. Patients with an incidence had an average age of 65.4 years (SD 19.2), 79.9% of their treatments was for chronic pathologies, they presented polymedication (6.8 drugs/patient SD 4.5) were treated in the scope of primary care (90.6%) and 22.9% were integrated into the contribution group (IHC) 1 whereby they were supposedly vulnerable because of socio-economic reasons.
Table 1. Basal characteristics of the sample analyzed
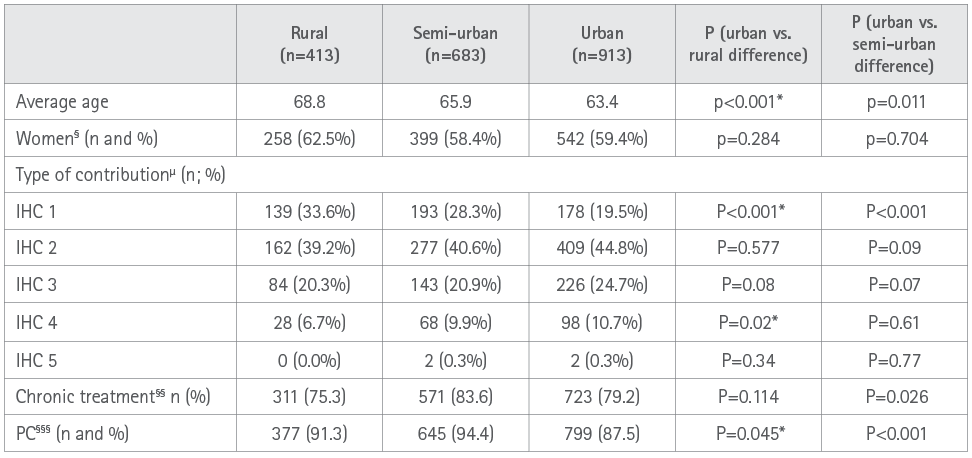
* A α =0.05 value is statistically significant.
§ Differences at 100% are values corresponding to men.
§§ Differences at 100% are values corresponding to acute treatments.
§§§ Differences at 100% are values corresponding to hospital treatment.
μ As in the remaining variables, the percentage of each variable is set out for each type of pharmacy.
PC: Primary Care.
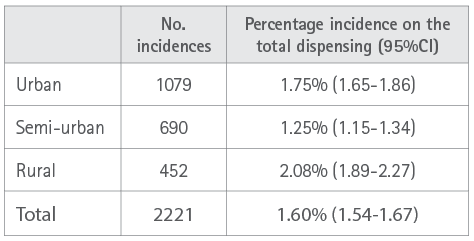
A total of 2221 incidences, equivalent to 1.6% (95%CI: 1.54-1.67) on the total dispensing and an average of 1.1 incidences per patient with incidences encountered, were detected. Analysis by subgroups revealed statistically significant differences (P<0.0001) according to the scope of the pharmacy. In the smaller populations a higher percentage incidence was observed that impacted the persistence of treatments (Table 2).
Table 2. Percentage incidence according to scope of the community pharmacy.
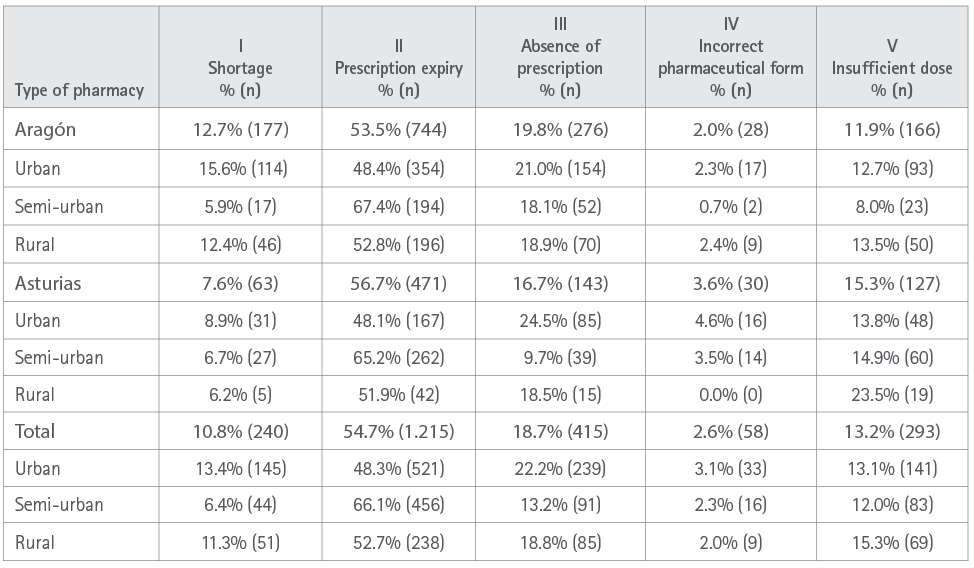
The incidence most commonly observed that restricted access to the pharmacological treatment was expiry of the prescription (54.7%; 95%CI=52.6-56.8), followed by absence of a valid prescription (18.7%; 95%CI: 17.1-20.3). Analysis according to the scope of the community pharmacy also revealed significant differences. It was observed that despite the higher proportion in a rural setting, the higher absolute number of incidences occurs in the urban setting due to its greater population (Table 3).
Table 3. Distribution of incidences associated with health system related risk factors according to social scope and by autonomous community (%, n)
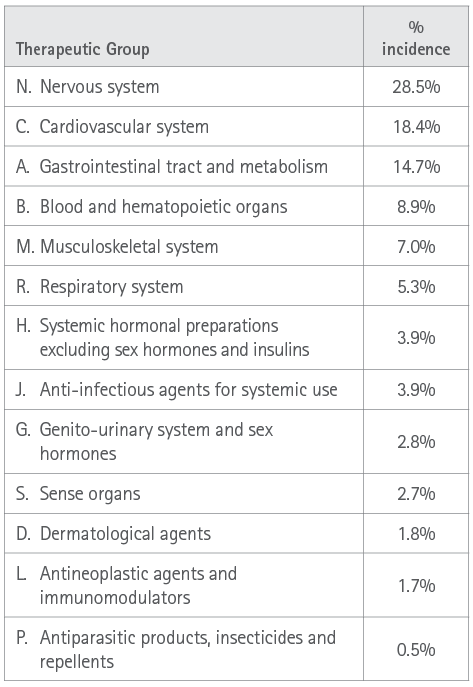
Finally, the pharmacological groups most commonly associated with the incidence of any risk factor leading to a break in persistence with the treatment (Table 4) were groups N (nervous system), C (blood and hematopoietic) and A (gastrointestinal tract and metabolism).
Table 4. Percentage incidence of the therapeutic groups at issue
DISCUSSION
The results of this study reveal the importance of the new health system related risk factors reported. These can lead to reducing adherence and/or the persistence of chronic treatments. It is observed that the problem has a greater impact on more vulnerable patients. The typical patient is an elderly person (65.4 years) with chronic pathologies and poly medicated (6.8 medicines) and a risk of socio-economic difficulty due to belonging to group IHC 1. This problem is also more markedly observed in the scope of the pharmacy located in the smallest municipalities. The combination of frail patient and small municipality may lead to more risk of lack of persistence; which might lead to a greater likelihood of reduction of attaining therapeutic aims and worsening of the patient’s health outcomes and quality of life as shown by the studies cited (3-5). These worse outcomes may also lead to a higher opportunity cost for the Health System. Various health resources must be used to tackle the consequences arising from these processes. Shortage is a growing problem as shown by the AEMPS report that detects a 31% increase over the first semester of 2022 compared to semester 2 of 2021 (10). For most of the incidences detected, a similar action to that of exceptional dispensing recently authorized for the case of amoxicillin in children, would minimize the reduction of the persistence of treatments. This would contribute to optimizing health outcomes (11).
The community pharmacy may collaborate in the resolution of this problem evaluated by means of the capacity for suitable drug replacement. First, according to amending the pharmaceutical form or the dose and also based on being able to dispense treatments that for merely administrative reasons hinder this as performed in several developed countries (Canada [13], United Kingdom [14], Australia [15]). An authorization of such aims would enable reducing the impact of health related risk factors by means of exceptional dispensing, whenever the incidence of such factors gradually increases currently (10).
To the best of our knowledge, this is the first multiregional study performed in our country that reveals and quantifies new health related risk factors in regard to adherence and/or therapeutic persistence. Whilst modifiable, this currently entails a health problem that gradually increases in the health systems of many countries.
Nonetheless, the study has some limitations. One: the geographic area has been restricted only to two Autonomous Communities as this is a pilot study; to resolve this aspect, a national study is being designed that analyzes this problem in depth to investigate ways to resolve this. Two: the quite small number of pharmacies incorporated into the study does not enable a rigorous extrapolation of the results all over the country. However, it has been sought to minimize this aspect as performing stratified probabilistic sampling might enable estimating differences because of geography as well as type of municipality. Three: the study time is reduced, which again interferes with the external validity. This is due to the aim of performing a fast exploratory study that gives suitable guidelines to subsequently perform the study on a national scale.
In light of the results it is suggested that further studies be performed that cover a wider geographic range, as well as studying different time spaces; with the aim of obviating seasonal bias and analyzing more in depth the drugs directly affected and the measures to resolve this.
CONCLUSIONS
The results obtained suggest that the current incidence of health system related risk factors in terms of adherence and/or persistence may comprise a health problem with a clinical, quality of life and financial impact. As these risk factors are modifiable, a greater capacity of community pharmaceutical action by means of exceptional dispensing to authorize the treatment’s continuity would be an acceptable solution.
ACKNOWLEDGEMENTS
To Ángel Sanz for his inestimable help and dedication to the project.
To all pharmacies taking part and researchers in both autonomous communities.
To STADA and SEFAC because performing the study was in part possible thanks to the financing attained by the awarding of the 8th STADA-SEFAC grant.
REFERENCES
1. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008 Jan-Feb;11(1):44-7. doi: 10.1111/j.1524-4733.2007.00213.x
2. World Health Organization. (2003). Adherence to long-term therapies : evidence for action. World Health Organization. Disponible en: https://apps.who.int/iris/handle/10665/42682
3. Dilla T, Valladares A, Lizán L, Sacristán JA. Treatment adherence and persistence: causes, consequences and improvement strategies. Aten. Primaria. 2009; 41(6):342–348. doi:10.1016/j.aprim.2008.09.031
4. Eichler HG, Abadie E, Breckenridge A, Flamion B, Gustafsson LL, Leufkens H, et al. Bridging the efficacy-effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nat Rev Drug Discov. 2011;10(7):495-506. doi:10.1038/nrd3501
5. Rafii F, Fatemi NS, Danielson E, Johansson CM, Modanloo M. Compliance to treatment in patients with chronic illness: A concept exploration. Iran J Nurs Midwifery Res. 2014;19(2):159-67. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4020025/
6. Prats Mas R (Coordinadora) Dispensación, adherencia y uso adecuado del medicamento. [Internet]. SEFAC. [último acceso 22/09/2022]. Disponible en: https://www.sefac.org/sites/default/files/2017-11/Adherencia_0.pdf
7. Sanz-Granda A, Satué de Velasco E. Análisis económico del ahorro de costes por sustitución de fármacos en Farmacia Comunitaria. [Internet] Madrid: SEFAC; 2016. [acceso 22/09/2022]. doi:10.33620/FC.2173-9218.(2023).PUB.6
8. Jaraiz I, Martínez AF, Satué E, Agustín E, López S, Allué JL. Proyecto sobre dispensación excepcional en el Principado de Asturias. Farm Comunitarios. 2023 Jan 02;15(1):5-12. doi:10.33620/FC.2173-9218.(2023).02
9. Procedimiento de actuación en las oficinas de farmacia andaluzaspara la dispensación excepcional por fin de tratamiento [Internet]. Consejo Andaluz de Colegios Oficiales de Farmacéutricos. [último acceso 20/12/22]. Disponible en: https://www.cacof.es/wp-content/uploads/2021/08/PROCEDIMIENTO-DE-ACTUACION-PARA-LA-DISPENSACION-EXCEPCIONAL-POR-FIN-DE-TRATAMIENTO.pdf
10. Informe semestral sobre la situación de problemas de suministro en España, primer semestre 2022. [Internet] Agencia Española de Medicamentos y Productos Sanitarios. [último acceso 20/12/2022] disponible en: https://www.aemps.gob.es/medicamentosUsoHumano/problemasSuministro/informes-semestrales/docs/primer-informe-semestral-2022.pdf
11. La AEMPS emite recomendaciones para paliar los problemas de suministro con las suspensiones pediátricas de amoxicilina 250mg/5ml [Internet]. Agencia Española de Medicamentos y Productos Sanitarios. 2022 [último acceso 20/12/22]. Disponible en: https://www.aemps.gob.es/informa/la-aemps-emite-recomendaciones-para-paliar-los-problemas-de-suministro-con-las-suspensiones-pediatricas-de-amoxicilina-250mg-5ml/
12. Satué E, Jaraiz I, Agustín E, Martínez A. Prescripción complementaria: ¿intrusismo o necesidad?. Farm Comunitarios. 2020 Mar 06;12(1):22-24. doi: 10.33620/FC.2173-9218.(2020/Vol12).001.04
13. P-10, r. 19.1 - Regulation respecting the extension or adjustment of a physician’s prescription by a pharmacist and the substitution of a medication prescribed. [Internet] Ministère de l’Emploi et de la Solidarité sociale. Quebec. [último acceso 23/12/22]. Disponible en: https://www.legisquebec.gouv.qc.ca/en/document/cr/P-10,%20r.%2019.1%20/
14. Supplementary Prescribing by Pharmacists. [Internet] Pharmaceutical Services Negotiating Committee. Londres. [Último acceso 29/12/22] Disponible en: https://psnc.org.uk/lpcs-and-local/locally-commissioned-services/en10-supplementary-prescribing-by-pharmacists/
15. Pharmacy Board position statement on pharmacist prescribing. [Internet] Australian Health Practitioner Regulation Agency. [Último acceso 29/12/22] Disponible en: https://www.pharmacyboard.gov.au/News/2019-10-15-position-statement.aspx

Editor: © SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria.
Copyright© SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria. This article is available from url https://www.farmaceuticoscomunitarios.org/. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en


