- The journal
- Midnight Navy / White , lower NIKE AIR JORDAN 1 MID SE DENIM CINNABAR HEMP-WHITE - lower Nike SB Kearny Cargo Pants - IlunionhotelsShops
- nike air force 1 uv color change da8301 100 101 release date
- Yeezys - Jordans, Musee-jacquemart-andre News, Jordan Essentials Statement Hoodie - release dates & nike.
- Air Jordan 1 Outlet Store
- 200 Release Date - nike gold air chukka moc high school - SBD - nike gold air yeezy glow in the dark sneakers boys Rattan Obsidian CZ4149
- Air Jordan 5 Racer Blue CT4838 004 Release Date Price 4
- adidas Yeezy Boost 350 V2 Onyx HQ4540 Release Date On Foot
- adidas y 3 qasa high triple black
- Air Jordan 1 Heritage 555088 161 Release Date On Foot
- adidas Ultra Boost 2022 COLD.RDY Magic Grey GZ0128 Release Date
- Aims & Scope
- Editorial Policies and Processes
- Scientific and Methodological Rigor
- Production and Administration
- Committees
- Authors
- Ethical Considerations
- Submit Article
- Archive
- Indexation
- Search
- Contact us
Farm Comunitarios. 2023 Jan 02;15(1):13-21. doi: 10.33620/FC.2173-9218.(2023).03
Study on the use of multi-compartment compliance aids to improve blood pressure values in hypertensive patients
INTRODUCTION
Arterial hypertension is the leading preventable cause for cardiovascular diseases and mortality worldwide (1). In Spain, 42.6% of the population of people >18 is hypertensive, being more frequent in men than in women. AHT rarely occurs alone and sometimes it is associated with other pathologies like dyslipidemia or diabetes that also increase the cardiovascular risk. AHT prevalence increases with age, thus >60% of people of 60 or older suffer from it (2).
At present, adults aged 65 or older represent 20% of the population according to the census in Spain (3). This population group represents two important characteristics strongly related: polypathology (e. i., simultaneous presence of 2 or more chronic diseases) and polymedication (taking 5 or more drugs for 6 months or more) (4).
In this regard, it has been demonstrated the existence of a relation between advanced age, polypathology and polymedication with an increase in the lack of adherence (5) responsible of multiple clinical and economic consequences derived from the increase of morbidity and mortality seen in non-adherent patients (6). In fact, reducing the lack of adherence to medication and specifically to the medication for AHT control is one of the objectives proposed by the World Health Organisation (WHO) since 2003 (7).
According to the National Analysis of Treatment Adherence in Chronic Pathologies, which analyses data of 12 chronic diseases, including AHT, 51.6% of Spanish patients are non-adherent to the treatments (8). In fact, although 88.3% of diagnosed hypertensive patients are prescribed with a drug to control AHT, only 30% of them have controlled arterial blood pressure, maybe due to a lack of treatment adherence (2).
In the last years, multiple interventions haven been conducted in order to improve these adherence data and different results have been obtained (7.9-14). A meta-analysis assessed 771 of these interventions conducted worldwide and the results suggested that those interventions conducted by community pharmacists (CF) significantly increased patient’s treatment adherence compared to those conducted by other healthcare professionals (15). Some of the interventions conducted by CF include implementation of multi-compartment compliance aids (MCAs), which not only allows improvement of treatment adherence but also prevents the occurrence of problems related to the medication (PRM) like taking the wrong drug or overdoses, frequent in polymedicated elderly people (16).
Elaboration of MCAs is a post-dispensing action conducted by the CF under its personal responsibility, regulated by Article 86.1 of the Royal Legislative Decree 1/2015 of 14 July by means of which the revised text of the Protection and Rational Use of Drug Products and Medical Devices Act is approved (17). Despite the potential improvement in adherence that can be obtained with the implementation of MCAs, only a few studies evaluating the improvement in adherence with this system have been found in the literature (18,19). Therefore, the objective of the analysis in this work was improvement in adherence of polymedicated patients with uncontrolled AHT by means of the implementation of MCAs compared to a control group, and it was measured by an improvement in patients’ arterial blood pressure (BP) values. Additionally, as supplementary objectives, other parameters were assessed, including knowing the degree of patient’s satisfaction with the MCA (assessed in a Likert scale in the last visit) as well as estimation and comparison of the cost of the described antihypertensive agents (these objectives will be described in detailed in the following publications).
METHODS
General Information
Multicentric epidemiological study with 35 community pharmacies that were randomised to the intervention group (MCA group) or the control group and which made a follow-up on the patients for 6 months. Randomisation was made by grouping; e. i., each of the pharmacies included all its patients either in the control group or in the MCA group as per previous allocations. For the allocation of each pharmacy to the corresponding group, the pharmacies with similar population characteristics (neighbourhood, rural, urban or coast) were matched by pairs so that after randomisation one of them will be in the control group and the other in the intervention group (18 in the MCA group and 17 in the control group).
Study Inclusion/Exclusion Criteria
To be included either in the control group or the intervention group of the study, patients should meet the following criteria:
1. Adult patient aged 55 years old.
2. Polymedicated patient (taking >6 drugs daily uninterruptedly over a period of ≥6 months).
3. Patient receiving electronic prescription since at least 3 months.
4. Patient with AHT, not controlled at the baseline visit and with treatment for over three months
It is considered uncontrolled AHT (1) when:
a. Systolic blood pressure (SBP)/diastolic blood pressure (DBP) levels are above 140/90 mmHg.
b. Limits of controlled SBP/DBP must be inferior to 130/80 when given the following circumstances: a previous cardiovascular event, stablished nephropathy, diabetes, patients with ≥3 cardiovascular risk factors (male, mean age 55 years old for men or 65 years old for women, smoking, dyslipidemia, family history of premature cardiovascular disease), heart failure or patients with metabolic syndrome.
Blood pressure must be measured with a sphygmomanometer validated by the European Society of Hypertension (ESH) and 2-3 measurements must be obtained in adequate conditions according to established international guidelines (1,20).
Study exclusion criteria:
1. Patients receiving antipsychotic agents.
2. Patients with any physical or mental disability preventing them from going to the community pharmacy and taking part in the project.
3. Patients who are already receiving the MCA preparation service.
The pharmacist counted the non-taken tablets found in the MCA the patient returned, and the patient’s treatment adherence was estimated (it was considered a non-adherent patient if the adherence was below 80%).
Adherence was assessed using a version of Morisky-Green test (Annex) (21,22) applied in every visit (baseline visit, Month 1 visit, Month 3 visit and Month 6 visit).
Annex. Morisky-Green Test
Study Development
FIRST VISIT
The following data were obtained:
- Sociodemographic variables: age, sex, level of education, province/state/county, country of origin.
- Number of hospital admissions during the previous year. These data were obtained by asking the patient.
- Cardiovascular risk factors, diabetes mellitus, previous acute myocardial infarction, substance abuse, nephropathies, dyslipidemia, cardiac illness, strokes, weight, and size. These data were obtained by asking the patient.
- Active principle (antihypertensive or non-antihypertensive agent) the patient takes based on the electronic prescription information and asking directly to the patient.
- Blood pressure measurement (3 measurements as per the recommendations of the ESH Guidelines), always with the same device and in the same time interval. The mean value of the three measurements was obtained.
- Patient had to present the results of recent lab tests and the total cholesterol and HDL data were documented.
- Patients had to fill out an adapted Morisky-Green test to assess the degree of baseline treatment adherence.
- Patients of the pharmacy randomised to the control group were given the medicine in the traditional way as the other patients of the pharmacy.
- All the patients (MCA group and control group) were provided standardised information about hypertension and signed the informed consent.
- Suspected adverse reactions were assessed and documented.
SECOND AND SUCCESSIVE VISITS
(Month 1 and Month 3)
Both groups
- Blood pressure measurement (3 measurements as per the ESH recommendations).
- Adapted Morisky-Green test.
- Measurement changes were documented.
- Suspected adverse reactions were documented.
In the group with MCA intervention
- Medication with MCA was dispensed.
LAST VISIT (Month 6)
Both groups
- Adapted Morisky-Green test.
- An evaluation of HDL level was made using the cobas b 101 system (Roche Diagnostics) for those patients who had no lab tests at the end of the study.
- Blood pressure measurement (3 measurements as per the ESH recommendations).
- Measurement changes were documented.
- Suspected adverse reactions were documented.
- Pharmacist’s satisfaction survey.
In the group with MCA intervention
- Medication with MCA was dispensed.
- Assessment of patient’s satisfaction with the MCA system.
- Time spent dispensing the medication with the MCA system.
VISIT EVERY 15 DAYS
Only the group with MCA intervention
- Medication dispensing (MCA delivery and collection every 15 days for 6 month of follow-up).
Table 1 summarises the data collected in each of the visits carried out.
Table 1 Data obtained in each visit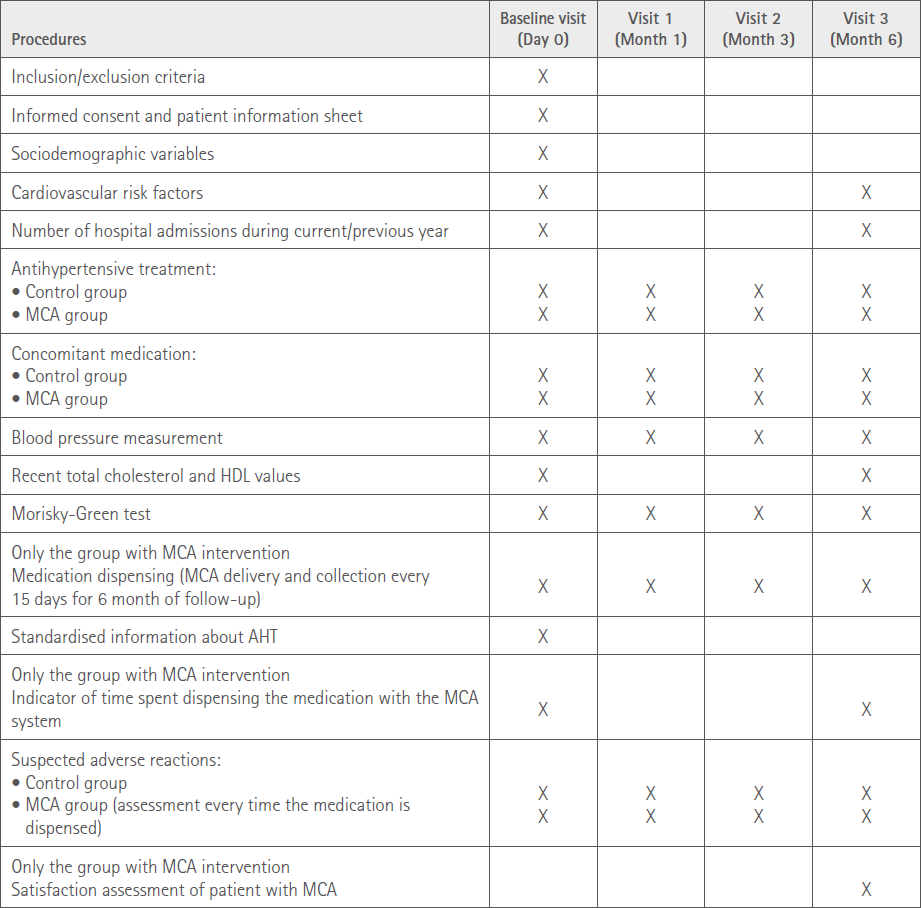
Sample Size Estimation
Since the intervention consisted of a medication preparation service that does not modify the antihypertensive treatment, but could only improve adherence, the impact that it could have on blood pressure was estimated to be less than that of comparative clinical trials with different active principles. For this reason, the sample size was estimated to detect a change of 8 mmHg between the systolic blood pressure and final blood pressure (at Month 6). Accepting a bilateral alpha error at 0.05 and a beta error at 0.2, 123 patients would be necessary in the first group and 123 patients in the second group to detect a similar difference or superior to 8 units (in mmHg) between baseline blood pressure value and final systolic blood pressure (SBP) value. An estimated common standard deviation would be 20. The estimated loss to follow-up rate was 20%.
The last final sample valid for the analysis was 88 patients in the MCA group and 107 in the control group.
Analysis
A descriptive analysis of all the variables was conducted and comparability between groups at baseline was evaluated. Blood pressure assessment was made by calculating the mean of the 3 measurements of sitting blood pressure for each patient. For the analysis of the primary variable, a covariance analysis was carried our taking the baseline measurement of blood pressure as the covariable, the intervention group as the independent variable and final measurement at Month 6 as the dependent variable (prior normality verification). Change of measurement at Month 3 was also evaluated. Continuous variables were categorised and the association between primary outcomes and independent variables were assessed using a univariate logistic regression analysis. Subsequently, all variables with a p < 0.05 were included in a multivariate model to estimate the odds ratio and the corresponding 95% confidence intervals. To do so a forward stepwise regression model of variables was used. Analysis was conducted with Statistical Analysis Software, version 9.4. Those in charge of the statistical analysis were blinded as to the patient’s identity and the group the patients were randomised to.
Study Classification and Evaluating ECCR
The study protocol was sent to the Spanish Agency of Medicines and Medical Products (AEMPS) for its classification and it was classified as an EPA SP study since it is a prospective follow up of patients, given that the primary factor of exposition is not a drug but the assessment of the clinical and economic impact of the implementation of the MCA service.
The study was approved by the regional ECCR of the Madrid Community of Castilla-La Mancha and Cataluña.
Ethical Aspects and Protection of Study Participants
The study was conducted in accordance with the requirements provided in the Declaration of Helsinki (Seoul revision, October 2008) and the current Spanish Legislation pursuant to the disposition in the Ministerial Order SAS/3470/2009 regarding observational studies. Treatment, communication, and transfer of personal data of all participants comply with the Organic Law 15/1999 of 13 September for the protection of personal information. As general considerations, all parties involved in the study accepted the national and international ethical rules for clinical research. An ECCR evaluated the protocol prior to patient inclusion to the study. Any data required by protocol was subjected to audits by the sponsor, independent organisations and/or competent authorities, but the confidentiality of the data was an indispensable condition in accordance with the previously cited law.
RESULTS
Participation and Follow-Up
A total of 35 community pharmacies participated in the study, 17 in the control group and 18 in the MCA, with a recruitment of 107 and 88 patients, respectively. All of the participants remained in the study till its completion and there were no significant differences between both groups. Participants mean age was 76.4 SD 8.9 and 57.4% were women. At the beginning of the study, no significant differences were seen regarding patient’s comorbidities; but patients included in the MCA group had a higher number of hospital admissions in the 12 months prior to the study. Comorbidities in each study group and the progression were similar in both groups as well as the hospital admissions. All these data are presented in Table 2.
Table 2 Summary of comorbidities/history and hospital admissions at baseline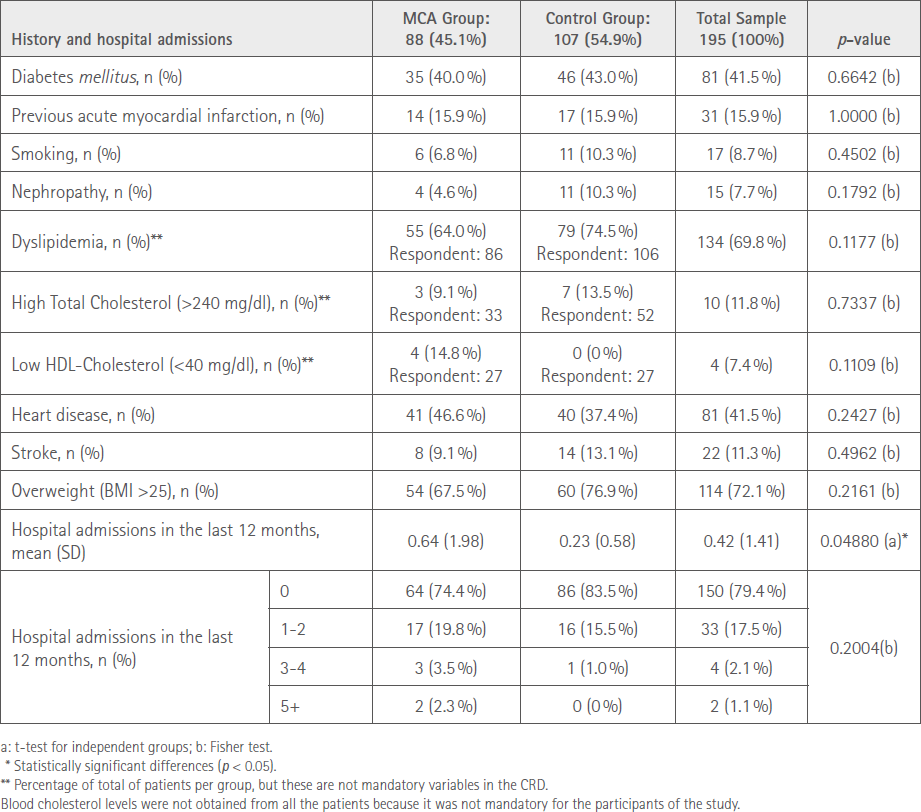
Adherence
At baseline, and according to the study screening criteria, 0% of patients were adherent to the medication. Adherence increased in both groups after the first visit (100%, 100% and 98.9% in the MCA group and 92.5%, 96.3% and 95.3% in the control group at Month 1, Month 3 and Month 6, respectively). Significant differences were seen only regarding adherence in the visit at Month 1 after study initiation (p-value between groups = 0.00087).
Treatments
Total number of prescribed treatments for BP decreased significantly during the study in the control group (8.5 vs. 7.8 between the first and the last visit; p = 0.0075) but not in the MCA group which remained constant (8.9 vs. 8.9; p = 0.9321). Non-antihypertensive medication increased significantly during the study in the MCA group (4.7 vs. 6.5), but not in the control group (5.3 vs. 5.6). With respect to the medication for AHT control, a significant decrease was observed in both groups, but not between groups (4.3 vs. 2.4 in the MCA compared to 3.1 vs. 2.3 in the control group).
Blood Pressure Progression
As can be seen in Table 3, BP decreased in both groups.
Table 3 Summary of mean BP values in the different groups during the study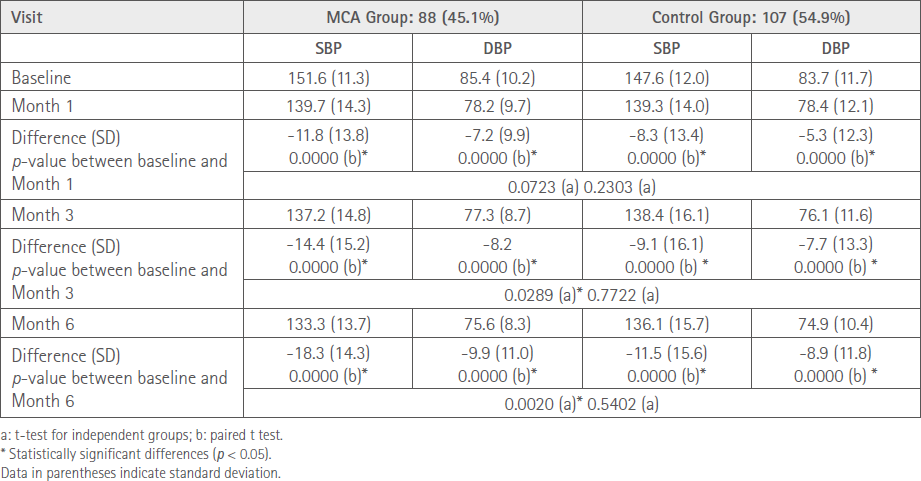
Patients in the MCA group presented a lower SBP of 18.3 mmHg vs. 11.5 mmHg in the control group from baseline to Month 6. Both data are statistically significant and the difference between groups is also statistically significant (p = 0.0020).
Similarly, patients in the MCA group presented a lower DBP of 9.9 mmHg vs. 8.9 mmHg in the control group from baseline to Month 6. Both data are statistically significant and the difference between groups is not statistically significant.
47.7% of patients in the MCA group reached blood pressure control at Month 6 compared to 39.3% in the control group. A significant increase was seen in the MCA group (Table 4).
Table 4 Progression of blood pressure control per study group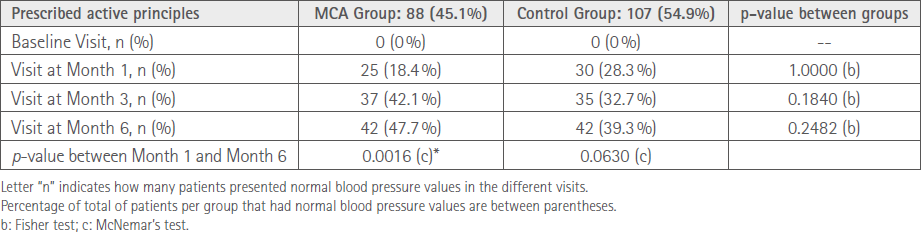
DISCUSSION
This work shows controversial results regarding our initial hypothesis. On the one hand, both the control group and the MCA group showed a decrease in the BP levels (SBP and DBP), though it was significantly higher in the MCA group. This can be so for several reasons. Firstly, some studies have demonstrated that the correct dispensation by the CF (providing information about the medicine, the pathology and healthcare education) as well as conducting pharmacist-patient interviews, improves patients BP levels compared to those who only receive the medication without any further information (9,15). Another fact that could also be responsible for this BP decrease is the regular measurement of blood pressure, measured in both groups at least 4 times and that has become one factor influencing BP level reduction (11), maybe patients being more aware that their BP values are not appropriate decide to take the medication to compensate for this. On the other hand, another factor that seems decisive in this work is the increase of adherence levels; however, despite both groups have reached practically the same levels of adherence, BP values in the MCA group are significantly lower than in the control group. This may be due to the different way in which adherence levels have been evaluated in both groups. In the MCA group, levels of adherence have been assessed by objective methods like counting the medication in the returned MCA, while in the control group, only the adapted Morisky-Green test has been used, a test that even though it’s validated, it’s not effective detecting lack of adherence like direct methods (23,24).
The implementation of the MCA system and the pharmaceutical intervention derived from its preparation improves the control of different chronic diseases evaluated in different scientific literature (25) and of the SBP levels as it was demonstrated in this work; however, further studies are necessary. Another factor that favours the use of the MCA system is that they are cost-effective. In our work, the cumulative cost for antihypertensive medication was approximately 100 euros less in the MCA group than in the control group (201.03 € in the control group vs. 109.34 € in the MCA group), fact that was demonstrated in multiples works (19,26,27). What seems undebatable is that the use of the MCA system has been postulated as an easy-to-use tool that improves patients’ life making them more autonomous and improves their perception about their health status, and at the same time decreases side effects associated with the medication (28,29).
ACKNOWLEDGEMENTS
This project was conducted thanks to the collaboration of the following community pharmacists to whom we thank for their participation: Arenas Benítez, I.; Cámara, R.; Carpintero Lozano, J.L.; Carreras Font, M.; Catalá Cerdá, A.; Chacón Hernández, J.; De Diego Colilla, V.; De Diego Martínez, C.; Dorca García, M.; Ferreres Gimeno, M.; Gallego Rivas, M.M.; Gil Sáenz, E.; Girones Saderra, M.; González Escanilla, S.; Gutiérrez Muñoz, L.; López-Grado Vela, S.; Manzano García. M.; Martín, N.; Martínez Catalá, N.; Méndez Mora-Figueroa, P.; Merencio Naudin, E.; Mestres Pedret, J.; Murillo Moya, L.; Nasarre Begueria, N.; Palomeque Fernández, JL.; Pruja Mach, M.D.; Purgimon Feliu, JL.; Rigola Garrofe, D.; Santaeugenia, MR.; Selva, C.; Sierra Alcañiz, I.; Simo Mas, R.; Sotoca Orgaz, M.; Vega Viejo, P. y Xalabarder Angli, A. The authors want to thank specially to Montserrat Iracheta Todó for her special participation in the realization of this project.
REFERENCES
1. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018 Dec;36(12):2284–309. doi:10.1097/HJH.0000000000001961
2. Menéndez E, Delgado E, Fernández-Vega F, Prieto MA, Bordiú E, Calle A, et al. Prevalencia, diagnóstico, tratamiento y control de la hipertensión arterial en España. Resultados del estudio Di@bet.es. Rev Esp Cardiol. 2016;69(6):572–8. doi:10.1016/j.recesp.2015.11.036
3. Instituto Nacional de Estadística. [Internet]. 2020. Available at: https://www.ine.es/ss/Satellite?L=es_ES&c=INESeccion_C&cid= 1259926457058&p=1254735110672&pagename=ProductosYServicios%2FPYSLayout¶m1=PYSDetalle¶m3=1259924822888
4. Núñez Montenegro AJ, Montiel Luque A, Martín Aurioles E, Torres Verdú B, Lara Moreno C, González Correa JA. Adherencia al tratamiento en pacientes polimedicados mayores de 65 años con prescripción por principio activo. Aten Primaria. 2014;46(5):238–45 doi:10.1016/j.aprim.2013.10.003
5. Leslie KH, McCowan C, Pell JP. Adherence to cardiovascular medication: a review of systematic reviews. J Public Health (Oxf). 2019 Mar;41(1):e84–94. doi:10.1093/pubmed/fdy088
6. Dilla T, Valladares A, Lizán L, Sacristán JA. Treatment adherence and persistence: Causes, consequences and improvement strategies. Aten Primaria. 2009;41(6):342–8. doi:10.1016/j.aprim.2008. 09.031
7. Kisa A, Sabaté E, Nuño-Solinís R. ADHERENCE TO LONG-TERM THERAPIES : Evidence for action. 2003. Available at: https://www.researchgate.net/publication/318679616_ADHERENCE_TO_LONG-TERM_THERAPIES_Evidence_for_action/link/59773ebb0f7e9b4016c36bbb/download
8. Viatris. Mapa de la adherencia al tratamiento en España [Internet]. Available at: https://www.viatrisconnect.es/-/media/Project/Common/ViatrisConnectES/pdf/MAPA-DE-LA-ADHERENCIA.pdf
9. Kini V, Ho PM. Interventions to Improve Medication Adherence: A Review. Vol. 320, JAMA. United States; 2018. p. 2461–73. doi:10.1001/jama.2018.19271
10. Xu H-Y, Yu Y-J, Zhang Q-H, Hu H-Y, Li M. Tailored Interventions to Improve Medication Adherence for Cardiovascular Diseases. Front Pharmacol [Internet]. 2020 Nov 13;11:510339. doi:10.3389/fphar.2020.510339
11. Izeogu C, Kalinowski J, Schoenthaler A. Strategies to Improve Adherence to Anti-Hypertensive Medications: a Narrative Review. Curr Hypertens Rep. 2020 Nov;22(12):105. doi:10.1007/s11906-020-01115-4
12. Elnaem MH, Irwan NA, Abubakar U, Syed Sulaiman SA, Elrggal ME, Cheema E. Impact of Medication Regimen Simplification on Medication Adherence and Clinical Outcomes in Patients with Long-Term Medical Conditions. Patient Prefer Adherence. 2020;14:2135–45. doi:10.2147/PPA.S268499
13. Moise N, Schwartz J, Bring R, Shimbo D, Kronish IM. Antihypertensive drug class and adherence: an electronic monitoring study. Am J Hypertens. 2015 Jun;28(6):717–21. doi:10.1093/ajh/hpu199
14. Schwartz JK. Pillbox use, satisfaction, and effectiveness among persons with chronic health conditions. Assist Technol. 2017;29(4):181–7. doi:10.1080/10400435.2016.1219884
15. Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: Systematic review and meta-analysis. Prev Med (Baltim) [Internet]. 2017/03/16. 2017 Jun;99:269–76. doi:10.1016/j.ypmed.2017.03.008
16. Arco Ortiz de Zárate J, Saénz de Buruaga S, Núñez Babarro J. Sistemas personalizados de dosificación: funcionamiento. Farm Prof. 2008;22(1):36–40. Available at: https://www.elsevier.es/es-revista-farmacia-profesional-3-articulo-sistemas-personalizados-dosificacion-funcionamiento-13114982
17. Real Decreto Legislativo 1/2015, de 24 de julio, por el que se aprueba el texto refundido de la Ley de garantías y uso racional de los medicamentos y productos sanitarios. Boletín Oficial del Estado nº 177, (25/07/2015). Available at: https://www.boe.es/buscar/act.php?id=BOE-A-2015-8343
18. Llaves García E, Segura Beltrán MM, García-Jiménez E, Baena Parejo I. [Personalised Medication Dosage Systems for treatment compliance in patients with hypertension and dyslipidaemia]. Vol. 41, Atencion primaria. 2009. p. 472–3. doi:10.1016/j.aprim.2008.12.004
19. Serra-Prat M, Bartolomé Regué M, Fité Novellas B, Agustí Maragall C. [Efficacy of a Personalised Dosage System (PDS) in improving compliance with therapy of elderly on multiple medication]. Vol. 37, Atencion primaria. Spain; 2006. p. 524–6. doi:10. 1157/13089090
20. Gijón-Conde T, Domenech M, Bellver O, Luque R. Presión arterial. Farm Comunitarios [Internet]. 2022;14(Supl 2. Especial HTA SE-Artículos):5–12. doi:10.33620/FC.2173-9218.(2022).HTA.001
21. Rodríguez Chamorro MÁ, García-Jiménez E, Amariles P, Rodríguez Chamorro A, José Faus M. Revisión de tests de medición del cumplimiento terapéutico utilizados en la práctica clínica. Aten Primaria. 2008;40(8):413–7. doi:10.1157/13125407
22. Baturone MO, Al. (coordinador) et al. Atención a pacientes pluripatológicos [Electronic resource]: proceso asistencial integrado. Consejería de Salud Junta Andalucía. 2018. Available at: https://www.juntadeandalucia.es/sites/default/files/2020-04/1337162989aten_pluri.pdf
23. Pareja-Martínez E, Esquivel-Prados E, Martínez-Martínez F, García-Corpas JP. Questionnaires on adherence to antihypertensive treatment: a systematic review of published questionnaires and their psychometric properties. Int J Clin Pharm. 2020 Apr;42(2):355–65. doi:10.1007/s11096-020-00981-x
24. Val Jiménez A, Amorós Ballestero G, Martínez Visa P, Fernández Ferré ML, León Sanromà M. [Descriptive study of patient compliance in pharmacologic antihypertensive treatment and validation of the Morisky and Green test]. Aten primaria. 1992 Oct;10(5):767–70.
25. Mud Castelló F, Mud Castelló S, Mud Castelló MJ, Signes Mut A, Muñoz Ballester P. Colaboración de un centro sociosanitario y dos farmacias comunitarias en la revisión de la medicación y elaboración de sistemas personalizados de dosificación. Farm Comunitarios. 2018;10(2):15–20. doi:10.5672/FC.2173-9218.(2018/Vol10).002.03
26. Llaves García E, Segura Beltrán MM, García-Jiménez E, Baena Parejo I. Personalised Medication Dosage Systems for treatment compliance in patients with hypertension and dyslipidaemia TT - Sistemas personalizados de dosificación en el cumplimiento del tratamiento farmacológico de pacientes con hipertensión y dislipemias. Aten primaria [Internet]. 2009/07/08. 2009 Aug;41(8):472–3. doi:10.1016/j.aprim.2008.12.004
27. Cárdenas Valladolid J, María Mena Mateo J, Asunción Cañada Dorado M, Rodríguez Morales D, Sánchez Perruca L. Implantación y mejora de un programa de atención al mayor polimedicado en un área de atención primaria. Rev Calid Asist. 2009;24(1):24–31. doi:10.1016/S1134-282X(09)70072-7
28. Nunney J, Raynor DK, Knapp P, Closs SJ. How do the attitudes and beliefs of older people and healthcare professionals impact on the use of multi-compartment compliance aids?: a qualitative study using grounded theory. Drugs Aging. 2011 May;28(5):403–14. doi:10.2165/11587180-000000000-00000
29. Stewart D, Gibson Smith K, MacLeod J, Strath A, Paudyal V, Forbes-McKay K, et al. The experiences and beliefs of older people in Scottish very sheltered housing about using multi-compartment compliance aids. Int J Clin Pharm. 2018 Apr;40(2):394–402. doi:10.1007/s11096-017-0580-x
Cite this article as: Martín A, García-Pastor C, Iracheta M, Gómez JC, Tejedor-García N. Study on the use of multi-compartment compliance aids to improve blood pressure values in hypertensive patients. Farm Comunitarios. 2023 Jan 02;15(1):13-21. doi:10.33620/FC.2173-9218.(2023).03
Editor: © SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria.
Copyright© SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria. This article is available from url https://www.farmaceuticoscomunitarios.org/. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en


