- The journal
- adidas Basic Insulated Μπουφάν
- Adidas forum low ✨⭐🌟 кроссовки для города форум как форсы но от адидас, nmd r1 vs nmd r2 womans sizing pants suit , Украина #123590352, а не найк. форум — цена 2049 грн в каталоге Кроссовки ✓ Купить мужские вещи по доступной цене на Шафе
- SBD - nike blue air skylon 2 ebay auction site - White x nike blue huarache atomic pink foot locker shoes Low Brooklyn DX1419 - 300 Release Date , Off
- adidas Samba Sizing: How Do They Fit? , adidas nebzed k eh2542 negras , IetpShops
- Sneakers Draked Viola
- Air Jordan 1 Stage Haze Release Date 555088 108 1
- air jordan 1 mid crater grey
- Travis Scott Fragment Air Jordan 1 Low DM7866 140 Release Date 4
- nike dunk low university blue
- cheap nike sb dunk high new york mets cloud grey rush blue team orange white 2022 for sale dh7155 001
- Aims & Scope
- Editorial Policies and Processes
- Scientific and Methodological Rigor
- Production and Administration
- Committees
- Authors
- Ethical Considerations
- Submit Article
- Archive
- Indexation
- Search
- Contact us
Farm Comunitarios. 2023 Apr 14;15(2):29-40. doi: 10.33620/FC.2173-9218.(2023).15
Strategy to optimize patient safety in the community pharmacy dispensing service. Proposed checklists
INTRODUCTION
Patient safety is defined in accordance with the World Health Organization (WHO) as the health treatment discipline, whose aim is to prevent and reduce risks, errors and injuries suffered by patients during the provision of healthcare (1,2).
In accordance with incidence studies on medication errors made in a global setting, we can conclude that up to 80% of errors related to diagnosis, prescription and use of medicines could be prevented (3-5).
Similarly, if we focus on national terms, the estimated cost for the Spanish National Health System in accordance with the latest Spanish Ministry of Health document on the patient safety strategy 2015-2020, is approximately 1.8 billion euros, which represents almost 3% of Spanish Health System health expenditure (6).
According to Spanish Law 29/2006 of 26 July, on guarantees and rational use of medicines, when dispensing pharmacists “will ensure compliance with the guidelines set out by the patient’s attending physician for prescription, and cooperate with him during treatment follow-up by means of pharmaceutical treatment procedures, which contribute to ensure their efficacy and safety” (BOE-A-2006-13554). It is, therefore, the pharmacist’s responsibility to contribute to the safety of medication (7).
The Council of Europe presented resolution CM/Res (2020) that enables health authorities from all over Europe to implement pharmaceutical treatment (8). The main aim of all health professionals involved in the medication process must be to improve the patient’s quality of life. Therefore, collaboration between health professionals is essential to improve the population’s health outcomes. Pharmacists can contribute to the integral control of pharmacotherapy in coordination with other health professionals. Among the activities to perform, by means of the process of pharmaceutical treatment, the detection of problems related to pharmacotherapy, such as contraindications, duplicates, prescription errors, interactions, etc. is notable (9). Community pharmacy is a safety filter to detect possible incidences and errors with medicines (3).
One way to attain better results in the field of patient safety might be setting up a Standardized Operating Procedure (SOP) with checklists for all processes in which an error might be made during the dispensing service.
Checklists as a safety tool originate from the world of aviation where verifying critical points before the aircraft takes off, serves to improve the trip’s favourable prognosis. These checklists in the world of aviation, were designed to markedly reduce air mortality (10,11), which led to them being incorporated into the health system by the WHO in 2008. They reveal the critical points that must be considered in operating theatres under the maxim “Safe Surgery Saves Lives” on five different levels:
• Organizational.
• Checklist.
• Individual.
• Technique.
• Implementation.
The pilot studies on Checklists in Intensive Care Units (ICU) managed to reveal a marked statistical significance in mortality rates with a positive balance in terms of their usefulness by means of (10):
• Minimizing errors.
• Minimizing the risk of infections.
• Teamwork.
This positions checklists as a tool transposable to the field of primary care and community pharmacy. These are key aspects to favour quality and safe dispensing of the various pharmaceutical forms (12).
Checklists whilst dispensing medicines with complex pharmaceutical forms, with specific use and handling, may be a tool to optimize patient safety that can be explored (13).
After an exhaustive search, no publications were found on the use of checklists on transdermal patches, modified release forms and orodispersible tablets during the dispensing service in community pharmacy. Only publications in health services were found from a general point of view. Therefore, we have before us a pioneering initiative in the field of community pharmacy patient safety.
The Patient Safety Work Group (PSWG) of the Spanish Society of Clinical, Family and Community Pharmacy (SEFAC), comprised of 35 pharmacists, considered working on a safety project related to checklists during the dispensing of some complex pharmaceutical forms.
MATERIALS AND METHODS
The following strategy was used to conduct this project:
A team of 10 community pharmacists belonging to the cited work group was set up.
A brainstorming session took place to define the work topic. It was concluded that checklists would be prepared during dispensing of three complex pharmaceutical forms: transdermal patches, modified release forms and orodispersible forms. A bibliographical search was performed in PubMed, Google Scholar, Cochrane and Scielo using “Patient Safety” as a search term between January and November 2022.
A total of 35, 20, 11 and 125 papers were obtained in PubMed, Google Scholar, Cochrane and Scielo, respectively. Results were entered into the Zotero platform.
By means of a shared online document and a series of online Zoom meetings, a compendium of good practices was prepared for each pharmaceutical form (patches, modified release forms and orodispersible tablets), as well as a table with questions to pose during the dispensing service. Brainstorming initially took place, a bibliographic search was performed on the topics proposed and references were selected by the group’s members. Subsequently, the PSWG coordinator was responsible for giving form to the document’s content. This Checklist SOP aims to serve to optimize each community pharmacy’s dispensing protocols on safety. Compendia were prepared using information compiled in the bibliographic search by means of preparing own and copyright-free images.
PROPOSED CHECKLISTS
The SEFAC PSWG has designed a series of infographics as a compendium and checklists to work with them during the dispensing service. This is to optimize provision of the pharmaceutical forms studied in the community pharmacy dispensing service. It is thus aimed to visually strengthen the key steps shown in the checklists during the dispensing of these pharmaceutical forms, which serve as support to the community pharmacist (14-16).
Moreover, to perform this project from the CP, the outline of the methodology proposed by Pharmaceutical Care Forum in Community Pharmacy (Foro AF-FC) and the practical guide for Pharmaceutical Services in the Community Pharmacy to perform the Dispensing Service will be followed (17).
“Therefore, the pharmacist, in the event of a request for a medicine and after systematically verifying that the applicant for dispensing is the patient or carer, and the latter have sufficient information for their effective and safe use, verifies with the available information that the medicine is suitable for this patient, complies with the prevailing regulations and dispenses or does not dispense, together with the necessary information for optimal use.”
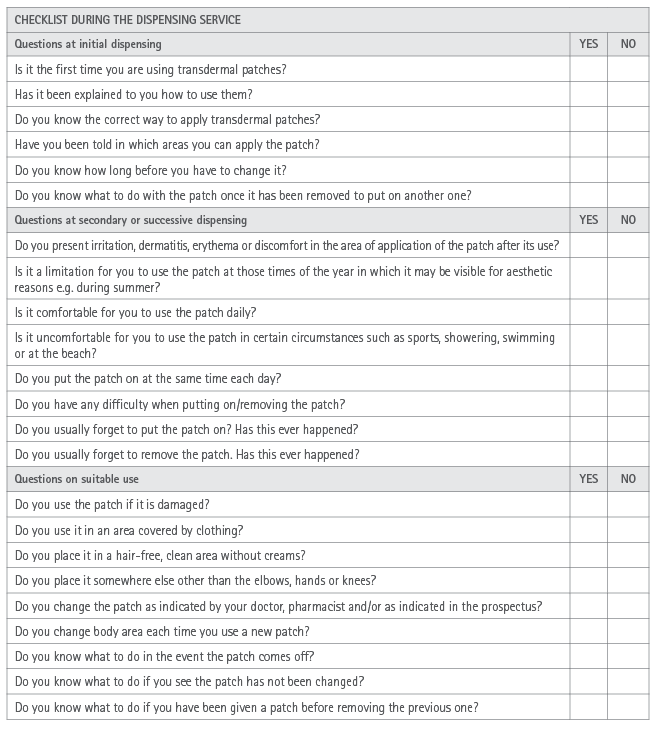
Transdermal patches (Appendix 1 and 2)
Transdermal therapeutic systems (TTS) or transdermal patches are pharmaceutical forms whose topical application enables dosing the medicines continuously transferred at a scheduled speed and for a set period of time, such that a systemic or specific action is obtained on a determined organ or system (18,19).
Advantages
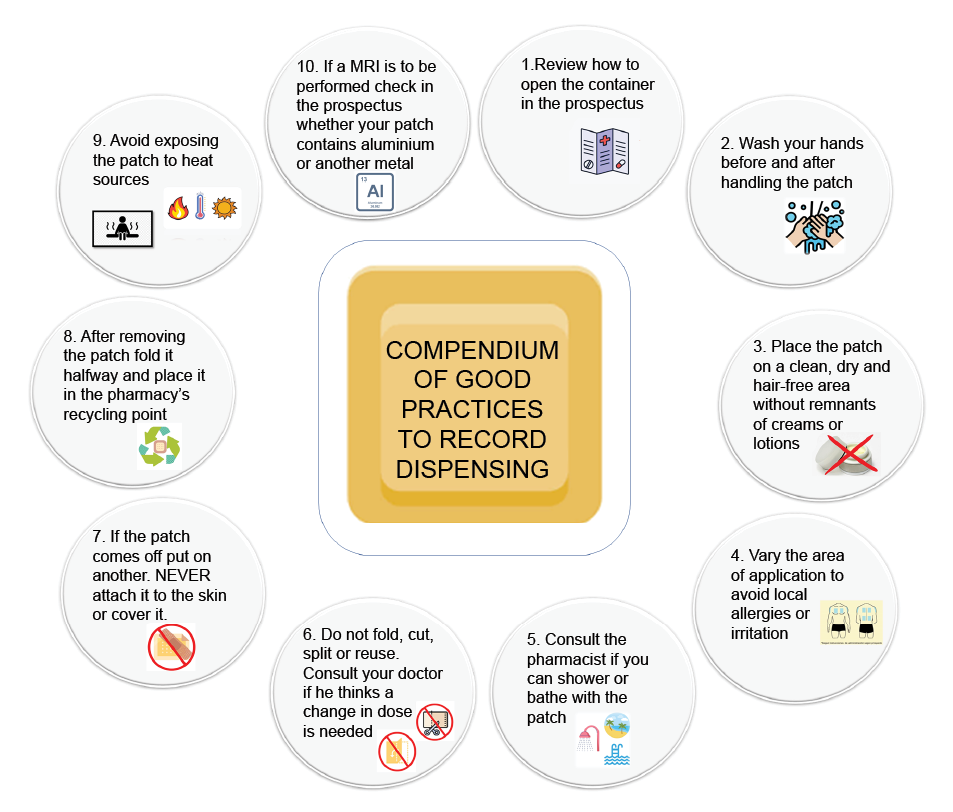
• They are especially useful for long term treatments as they facilitate detailed posological monitoring, with a constant, sustained and controlled release of the active substances that comprise them. Therefore, it must be guaranteed that these systems favour release of the medicine through the skin up to the bloodstream (19,22).
• Transdermal patches enable administering a broad variety of medicines, such as those used in hormonal therapy and hormonal contraception, among others (23). New uses such as insulin transdermal patches for diabetes patients, which enable improving acceptance (reducing the stress caused by the injection itself) and safety during dosing (controlled insulin secretion by means of determining blood pH and considering weight and the sensitivity of insulin supplied by each patient); avoiding errors and adverse effects, are also being investigated (24,25).
Disadvantages
It is important to verify that the posological recommendation for the patch is correct, that the patient knows how to use it, in which areas to place it, how to throw it away and ensure there is no incorrect use of the patch such as folding, cutting and/or using the same patch repeatedly (single use patch). Moreover, avoid direct exposure to sources of heat, which must be supervised during the patient’s pharmacotherapeutic follow up (20,25).
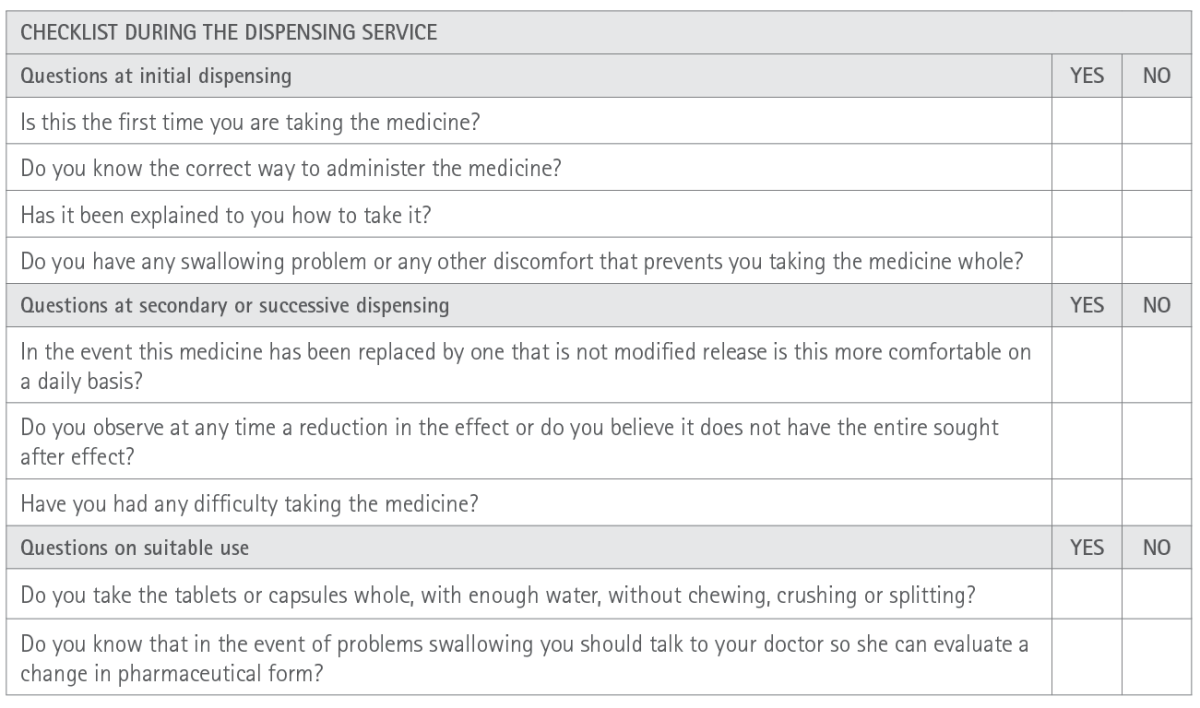
Modified release forms (Appendix 3 and 4)
The administration of oral medicines (tablets, capsules, powders, suspensions and solutions) is the most common, ideal and preferred method of administration, because of its convenience and safety compared to other methods (26).
Modified release pharmaceutical forms (MRF) are those designed such that the speed or place of release of the active substance change according to the immediate release pharmaceutical forms of the active substance itself (27).
Advantages
MRF enable medicines with a short action duration to be administered less frequently, thereby favouring therapeutic compliance. They usually entail improved pharmacokinetics of the active substance with increased bioavailability and a better toxicological profile; minimizing gastrointestinal adverse effects. In the specific case of opioids, MRF also reduce the risk of developing addiction because of lowering the maximum concentrations of opioids and increasing the times these are attained compared to immediate release forms. All these advantages mean that MRF are especially interesting for chronic diseases, in medicines with strict therapeutic compliance to maintain plasma concentrations within the limits of effectiveness and toxicity; for medicines absorbed quickly, are short duration or those medicines broken down in an acidic environment (27,28).
Disadvantages
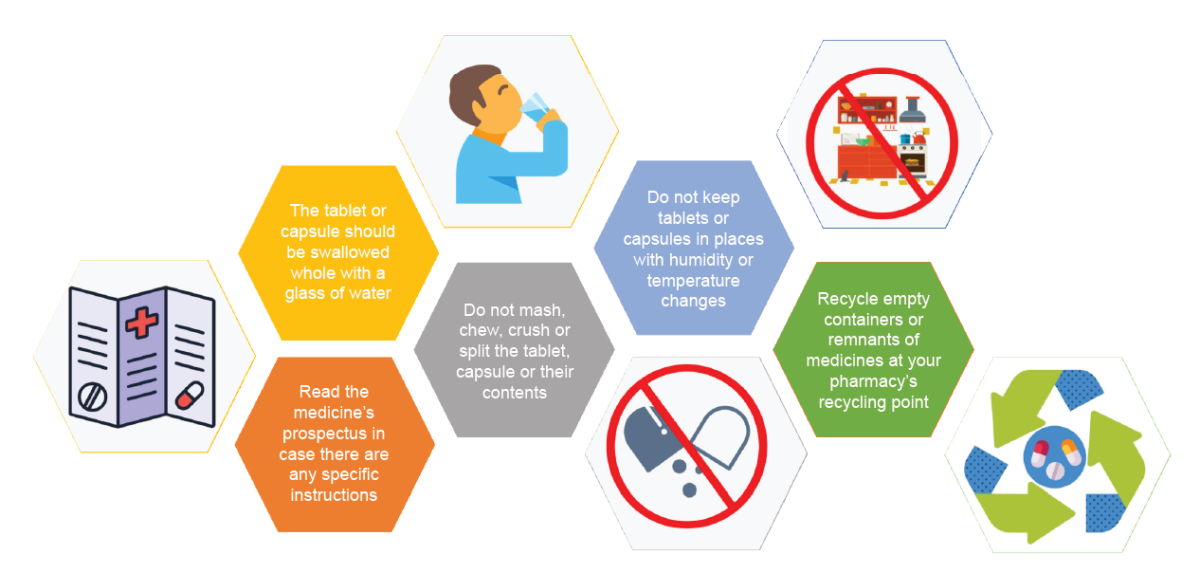
• Problems associated with incorrect handling. MRF usually present in the form of tablets or capsules that must not be administered chewed or crushed because of their potential toxicity and loss of long term effect. The patient should be warned that in general MRF should be swallowed whole. In the specific case of opioids, oral prolonged release pharmaceutical forms are highly susceptible to being altered as they contain a higher amount of active drug than immediate release formulations. Moreover, oral prolonged release pharmaceutical forms are much easier to handle than other pharmaceutical forms, such as transdermal patches. Exceptionally, some modified release tablets have a break groove and can be split. Some capsules of this kind contain microgranules, which enable them to be directly administered by tube or mixed with food if their integrity is maintained. There are no general rules and in each case the possibility of handling the medicine safely must be confirmed with the manufacturer and technical specification. The information on the possibility of splitting or crushing capsules or tablets is covered in the technical specifications and prospectus for the medicine (26-29).
• Exacerbation of overdose or onset of adverse reactions as a consequence of the prolonged action of medicines formulated as MRF.
• Pharmacological interactions with foods. Foods can alter the release of a medicine formulated as MRF.
Orodispersibles (Appendix 5 and 6)
Orodispersible tablets according to the Spanish Royal Pharmacopoeia are non-coated tablets aimed to be placed in the mouth, where they quickly disperse before being swallowed. They are characterized because they must be broken down in less than three minutes when subjected to the general breakdown test for tablets and capsules; that is, at a temperature 35ºC – 39ºC, in a liquid medium and in a tablet divider that complies with specifications (30).
Advantages (29-31)
• They combine the advantages of liquid forms and oral solid forms: they are accurate to dose and easy to swallow.
• They usually have a pleasant taste.
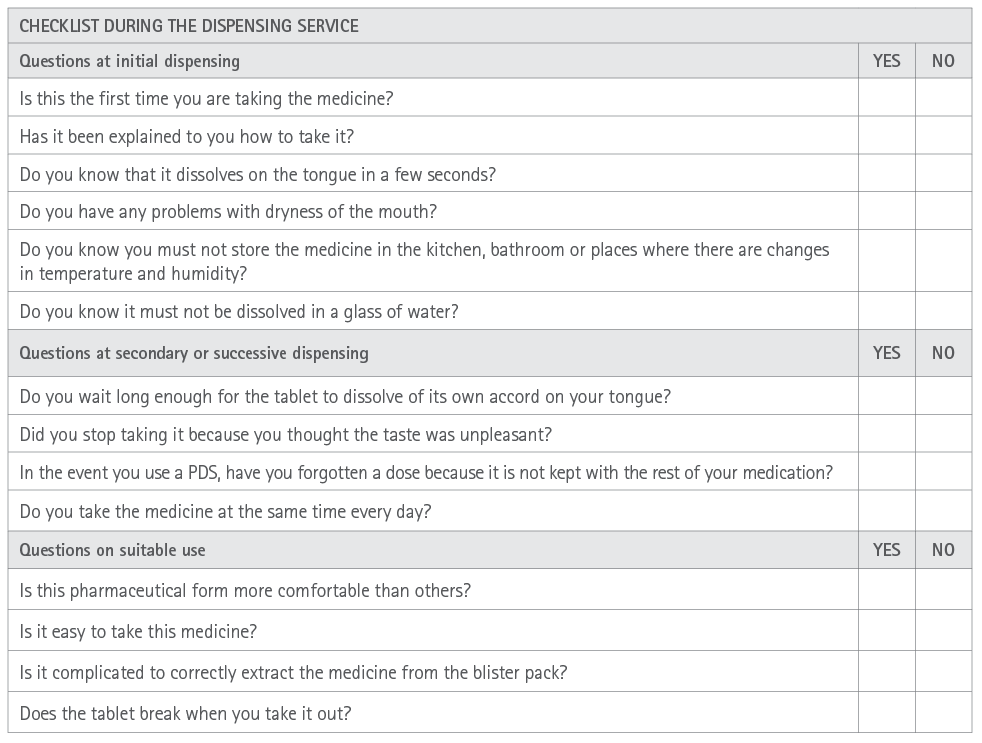
• The tablet does not need to be swallowed whole or with water as it dissolves quickly in saliva (1 to 3 minutes). This is especially an advantage for children, the elderly, Parkinson disease patients, people with dysphagia, phagophobia, mental disability and even patients treated with anti-psychotics.
• Improved bioavailability.
Disadvantages (30-33)
• They are more fragile. Due to the greater porosity they usually present that facilitates absorption of water inside, they lack the mechanical resistance of traditional tablets. Therefore, they are more susceptible to breakage.
• Excess humidity in the environment can lead to their physical instability.
• They cannot be incorporated into Monitored Dosage System (MDS).
• As they are a more novel pharmaceutical form, there is little knowledge by patients when they start to dissolve in the mouth.
• They are not recommended for patients with a lack of salivation as is the case in Sjögren syndrome, xerostomy and patients in treatment with anti-cholinergic medicines. These problems might be resolved to a certain extent by means of drinking a glass of water prior to taking the tablet.
APPLICABILITY
From the PSWG, a pilot study was performed in 20 pharmacies from different Autonomous Communities during 2023 (34). This enabled validating the applicability of these tools in the form of protocols. The ultimate aim is to attain optimal patient safety standards that can be simply and effectively applied from community pharmacy.
It is aimed that the detection, reduction and resolution of errors that might impact the patient’s safety reduce the associated health problems and rates of hospitalization and morbi-mortality because of medication errors.
In parallel, it is deemed appropriate to have a community pharmacist-general practitioner channel of communication to notify relevant points on patient safety that may arise during application of the SOP to checklists during the dispensing service.
ACKNOWLEDGEMENTS
We are especially grateful to the entire SEFAC Patient Safety Work Group.
REFERENCES
1. Silva Rodriguez CM. Evaluación de las buenas prácticas de prescripción y su relación con la dispensación de medicamentos en la farmacia de emergencia de un Hospital en el Callao, 2022. Repositorio Institucional – Universidad Cesar Vallejo [Internet]. 2022. Disponible en: https://repositorio.ucv.edu.pe/handle/20.500.12692/97748
2. World Health Assembly. Resolución WHA 72.6 por Acción mundial en pro de la seguridad del paciente 72.ª ASAMBLEA MUNDIAL DE LA SALUD Punto 12.5 del orden del día 28 de mayo de 2019. [Internet] 2019 [citado el 7 agosto de 2022]. Disponible en: https://www.who.int/es/news-room/fact-sheets/detail/patient-safety
3. Oñatibia-Astibia A, Aizpurua-Arruti X, Malet-Larrea A, Gastelurrutia MÁ, Goyenechea E. El papel del farmacéutico comunitario en la detección y disminución de los errores de medicación: revisión sistemática exploratoria. Ars Pharm [Internet] 2021; [citado el 7 de agosto de 2022] 62(1):15–39. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S2340-98942021000100015&lng=es
4. Romero GDL, Almiray SAL, Ensaldo CE. Intervenciones en la administración de medicamentos de alto riesgo. Rev CONAMED [Internet] 2020; [citado el 19 de agosto 2022] 25(2):95-97. Disponible en: https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=94393
5. Marufu TC, Bower R, Hendron E, Manning JC. Nursing interventions to reduce medication errors in paediatrics and neonates: Systematic review and meta-analysis. Journal of Pediatric Nursing [Internet]. enero de 2022 [citado 11 de febrero de 2023];62:e139-47. Disponible en: https://linkinghub.elsevier.com/retrieve/pii/S0882596321002578
6. Agra Varela Y. Coordinadora. Estrategia de Seguridad del Paciente del Sistema Nacional de Salud Período 2015-2020. Ministerio de Sanidad, Servicios Sociales e Igualdad Centro de Publicaciones. Madrid, 2016. [Internet]. [citado 19 de agosto de 2022]. Disponible en: https://seguridaddelpaciente.sanidad.gob.es/docs/Estrategia_Seguridad_del_Paciente_2015-2020.pdf
7. Ley 29/2006, de 26 de julio, de garantías y uso racional de los medicamentos y productos sanitarios. Boletín Oficial del Estado, número 178, (27 de julio 2006). Disponible en: https://www.boe.es/eli/es/l/2006/07/26/29/con
8. Consejo de Europa. Resolution CM/Res(2020)3 on the implementation of pharmaceutical care for the benefit of patients and health services [Internet]. [Citado 14 de febrero de 2022]. Disponible en: https://search.coe.int/cm/pages/result_details.aspx?objectid=090000 16809cdf26
9. Calvo Hernáez B, Gastelurrutia Garralda MÁ, Urionagüena de la Iglesia A, Isla Ruiz A, del Pozo Rodríguez A, Solinís Aspiazu MÁ. Oferta de servicios de atención farmacéutica: clave para un nuevo modelo de servicios de salud. Atención Primaria [Internet]. 1 de enero de 2022 [citado8 de febrero de 2023];54(1):102198. Disponible en: https://www.sciencedirect.com/science/article/pii/S0212656721002328
10. Maran E, Matsuda LM, Cavalcanti AB, Magalhães AMM de, Marcon SS, Haddad M do CFL, et al. Effects of multidisciplinary rounds and checklist in an Intensive Care Unit: a mixed methods study. Rev Bras Enferm [Internet]. 2022 [citado 19 de diciembre de 2022];75(3):e20210934. Disponible en: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-71672022000400185&tlng=en
11. Kupka JR, Sagheb K, Al-Nawas B, Schiegnitz E. Surgical safety checklists for dental implant surgeries—a scoping review. Clin Oral Invest [Internet]. 27 de agosto de 2022 [citado 19 de diciembre de 2022];26(11):6469-77. Disponible en: https://link.springer.com/10.1007/s00784-022-04698-1
12.Giovanni Ceccarelli, Emanuela Foglia, Lucrezia Ferrario, Pietro Nunnari. Care pathways production and review checklist: results from an HTA evaluation. Recenti Progressi in Medicina [Internet]. 1 de marzo de 2022 [citado 19 de diciembre de 2022];(2022March). doi:10.1701/3761.37483
13. Torres Y, Rodríguez Y, Pérez E. ¿Cómo mejorar la calidad de los servicios de salud y la seguridad del paciente adoptando estrategias del sector de la aviación? Journal of Healthcare Quality Research [Internet] 2022 [citado 13 de agosto de 2022]; 37:182-90. Disponible en: https://www.sciencedirect.com/science/article/abs/pii/S260364792 100107X
14. Adsuar G, Luque R, Grande E, Mera L, López M.D, Pérez R, et al. Optimización de la seguridad del paciente durante el servicio de dispensación de medicamentos con formas farmacéuticas tipo parche transdérmico. Farm Com. 2022 Jun 15;14(Supl 1. Congreso SEFAC):235. https://www.farmaceuticoscomunitarios.org/es/node/33
15. Adsuar G, Luque R, Grande E, Mera L, López M.D, Pérez R, et al. Optimización de la seguridad del paciente durante el servicio de dispensación de medicamentos con formas farmacéuticas tipo comprimido bucodispersable. Farm Com. 2022 Jun 15;14(Supl 1. Congreso SEFAC):236. https://www.farmaceuticoscomunitarios.org/es/node/3340
16. Adsuar G, Luque R, Grande E, Mera L, López M.D, Pérez R, et al. Optimización de la seguridad del paciente durante el servicio de dispensación de medicamentos con formas farmacéuticas de liberación modificada FLM. Farm Com. 2022 Jun 15;14(Supl 1. Congreso SEFAC):255. https://www.farmaceuticoscomunitarios.org/es/node/3389
17. Foro de Atención Farmacéutica-Farmacia Comunitaria (Foro AF-FC). Guía práctica para los Servicios Profesionales Farmacéuticos Asistenciales en la Farmacia Comunitaria. Madrid: Consejo General de Colegios Oficiales de Farmacéuticos; 2019. Disponible en: https://www.sefac.org/system/files/2021-02/AF_GUIA_SPFA_FORO_2021_ONLINE_PGs.pdf
18. Ramírez MA, Martínez ER, Zapara JR, Deveze MA, Ruiz AJ, Solorio CR. Sistemas Transdérmicos de administración de fármacos. Naturaleza y Tecnología.[Internet]2019 [citado 7 de agosto 2022]; 6(1):22-27. Disponible en: http://www.naturalezaytecnologia.com/index.php/nyt/article/view/345/pdf1
19. Avelar B, Laura L. Modelos in vivo e in vitro empleados para la determinación de la actividad antiinflamatoria de flavonoides y la tendencia para su aplicación en Sistemas Terapéuticos Transdérmicos (STTs). 6 de diciembre de 2022. Disponible en: http://riaa.uaem.mx/xmlui/handle/20.500.12055/2921
20. Latif MS, Nawaz A, Rashid SA, Akhlaq M, Iqbal A, Khan MJ, et al. Formulation of Polymers-Based Methotrexate Patches and Investigation of the Effect of Various Penetration Enhancers: In Vitro, Ex Vivo and In Vivo Characterization. Polymers [Internet]. enero de 2022 [citado 10 de febrero de 2023];14(11):2211. Disponible en: https://www.mdpi.com/2073-4360/14/11/2211
21. Mateos A, Mateos M. ¿Se puede administrar medio parche de fentanilo en una reducción gradual de dosis? A propósito de un caso. Farmaceúticos Comunitarios [Internet] 2020 [citado 7 de agosto 2022]; 12(Supl 2.Congreso SEFAC 2020):67. Disponible en: https://www.farmaceuticoscomunitarios.org/es/journal-article/se-debe-administrar-medio-parche-fentanilo-una-reduccion-gradual-dosis-proposito
22. Arunprasert K, Pornpitchanarong C, Rojanarata T, Ngawhirunpat T, Opanasopit P, Patrojanasophon P. Mussel-inspired poly(hydroxyethyl acrylate-co-itaconic acid)-catechol/hyaluronic acid drug- in-adhesive patches for transdermal delivery of ketoprofen. International Journal of Pharmaceutics [Internet]. 15 de diciembre de 2022 [citado10 de febrero];629:122362. Disponible en: https://www.sciencedirect.com/science/article/pii/S0378517322009176
23. Cortés-Bonilla M, Velázquez-Ramírez N. Anticoncepción transdérmica. Ginecol Obstet Mex [Internet] 2020[citado 7 de agosto 2022];88(Supl 1):S42-S46. Disponible en: https://ginecologiayobstetricia.org.mx/articulo/anticoncepcion-transdermica
24. Moreno MI, Ochoa RM, Orellana VM, Ruiz CA. La importancia de los parches de insulina para los pacientes diabéticos. ¿Utopía o Realidad?. Revista Científica de Investigación actualización del mundo de las Ciencias[Internet] 2019.[citado 7 de agosto 2022]: 3 (3): 82-106. Disponible en: https://reciamuc.com/index.php/RECIAMUC/article/view/268
25. Ayala G. Nuevos parches de insulina para la diabetes podrían reemplazar a las inyecciones de insulina para siempre . DIabetes AC. [Internet] 2022 [citado 13 agosto de 2022]. Disponible en: https://www.diabetes.ac/nuevos-parches-insulina-la-diabetes-podrian-reemplazar-a-las-inyecciones-insulina-siempre/
26. Mishra V, Nayak P, Yadav N, Singh M, Tambuwala MM, Aljabali AA. Orally administered self-emulsifying drug delivery system in disease management: advancement and patents. Expert Opin Drug Deliv. [Internet] 2021 [citado 7 de agosto 2022]; 18(3):315–322. doi:10.1080/17425247.2021.1856073
27. Pérez-López A, Gómez-Lázaro L, Martín-Sabroso C, Aparicio-Blanco J. Sistemas de liberación prolongada de opioides: Analgesia y dependencia. ANALES RANF [Internet] 2021 [citado 7 agosto 2022]. 87 (1):35-51. Disponible en: https://analesranf.com/articulo/8701_03/
28. Rojas MT, Mulas F, Gandía R, Ortiz P. (2022). Enfoque terapéutico del trastorno por déficit de atención e hiperactividad. Medicina (Buenos Aires) 2022; Vol. 82 (Supl. III): 51-56. Disponible en: https://www.medicinabuenosaires.com/revistas/vol82-22/s3/51s3.pdf
29. Gómez AE. Manipulación de especialidades farmacéuticas. Farmacia Profesional [Internet] 2017 [citado 7 de agosto 2022]; 21 (4): 44-48. Disponible en: https://www.elsevier.es/es-revista-farmacia-profesional-3-pdf-13102032
30. Aguilar M, Aranda C. Características de los pacientes con dolor musculoesquelético moderado a intenso tratados con comprimidos bucodispersables de paracetamol 325 mg/tramadol HCI 37,5 mg (Paxiflas®) respecto a otras formas orales de la misma combinación. Estudio PROPAX. Rev Soc Esp Dolor [Internet]. 2020 [citado 19 de diciembre de 2022]. Disponible en: http://gestoreditorial.resed.es/fichaArticulo.aspx?iarf=224682766-74923 4416274
31. Suraweera C, Hanwella R, De Silva V. Phagophobia: a case report. BMC Res Notes [Internet] 2014 [citado 7 de agosto 2022]; 7:574. Disponible en: https://pubmed.ncbi.nlm.nih.gov/25164031/
32. Ruiz A; Muñoz H. Composición farmacéutica bucodispersable de melatonina.[Internet]. España:Universidad de Granada/Hospital Real Cuesta Del Hospicio Granada; 2015 [citado 7 de agosto 2022]. Disponible en: https://digibug.ugr.es/bitstream/handle/10481/36393/ES2457718B1.pdf?sequence=1&isAllowed=y
33. Thomson WM, Smith MB, Ferguson CA, Moses G. The Challenge of Medication-Induced Dry Mouth in Residential Aged Care. Pharmacy [Internet]. 2021 Oct 1;9(4):162. 10.3390/pharmacy9040162
34. Quispe ML. Gestión de calidad en el proceso de manejo y control de medicamentos y dispositivos médicos. [Tesis para obtener el título de Químico-Farmacéutico]. Huancayo - Perú . Facultad de Ciencias de la Salud. Escuela Profesional de Ciencias Farmacéuticas y Bioquímica. Universidad de Roosevelt. [Internet]. 2022 [citado 11 de febrero 2022]. Disponible en: https://repositorio.uroosevelt.edu.pe/bitstream/handle/20.500.14140/947/Tesis%20Quispe%20-Tello.pdf?sequence=1&isAllowed=y
Appendix 1. Compendium of good practices to record the dispensing of transdermal patches
Appendix 2. Checklist during the dispensing service for transdermal patches
Appendix 3. Compendium of good practices to record the dispensing of modified release forms
Appendix 4. Checklist during the modified release forms dispensing service
Appendix 5 Compendium of good practices to record the dispensing of orodispersible tablets
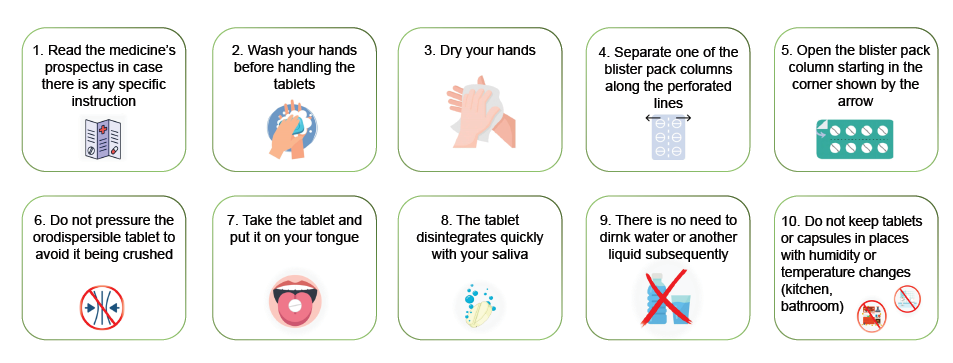
Appendix 6 Checklist during the dispensing of orodispersible tablets
Editor: © SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria.
Copyright© SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria. This article is available from url https://www.farmaceuticoscomunitarios.org/. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en


