- The journal
- Jordan Brand had the tough task of following up the widely successful , Air Jordan 1 Retro Low OG Black Dark Powder Blue UK 11 , Fenua-environnementShops Marketplace
- Украина #98312663 , nike running tank tops women cute , Nike air force 1 worldwide white/green женские кроссовки найк аир форс — цена 1700 грн в каталоге Кроссовки ✓ Купить женские вещи по доступной цене на Шафе
- 429 Too Many Requests
- air jordan 1 mid outlet
- Puma et Jeff Staple ont su nouer une solide relation durant ces dernières années , Buy & Sell Sneakers , AcmShops Marketplace
- adidas Yeezy Boost 350 V2 Onyx HQ4540 Release Date On Foot
- air jordan 4 white tech grey black fire red ct8527 100 release date
- NBA x Nike Dunk Low 75th Anniversary
- Gnarhunters Nike SB Dunk Low DH7756 010 Release Date On Foot
- nike dunk low university blue
- Aims & Scope
- Editorial Policies and Processes
- Scientific and Methodological Rigor
- Production and Administration
- Committees
- Authors
- Ethical Considerations
- Submit Article
- Archive
- Indexation
- Search
- Contact us
Farm Comunitarios. 17(2):48-59. doi: 10.33620/FC.2173-9218.(2025).14
Comprehensive Analysis of Compounded Prescription Orders: Types of Errors and Variability Among Autonomous Communities in Spain
INTRODUCCIÓN
A compounded prescription (CP) is a medication intended for a specific patient, prepared by a pharmacist who fulfills a detailed prescription of active ingredients (AIs) issued by a licensed healthcare professional when no commercially available medicine is suitable for the patient, and always based on scientific evidence. For this reason, it represents the final resort within the Spanish National Health System (SNS) in cases of drug shortages, or concerns related to the safety or efficacy of marketed medications (1–3). To avoid terminological confusion, official preparations are also included under CP.
In Spain, these medicines are regulated by Royal Decree (RD) 1718/2010 of December 17, RD 1/2015 of July 24, and RD 905/2003 of July 11 (1,4).
For a compounded prescription to be dispensable, the prescription must meet the following requirements, regardless of whether it is in paper or electronic format (4,5):
- Patient information: Full name, date of birth, and ID number (National Identity Document (DNI) or equivalent), or patient identification code for prescriptions within the SNS.
- Medication information: Name(s) of the active ingredient(s), dosage, pharmaceutical form, intended recipient (if applicable: infant, child, adult, etc.), route of administration, number of packages or amount of medication, dosage regimen, and duration of treatment.
- Prescriber information: Full name, contact details (telephone), professional address (including city and country), professional qualification, license number, and signature.
- Other data: Date of prescription, expected date of dispensing (in case of successive dispensations), and dispensing order number (if a planned dispensing date is indicated).
To date, only one study has assessed the actual compliance with these regulations in Spain (5). However, that study did not differentiate between commercial medications and compounded prescriptions. Furthermore, it focused solely on paper prescriptions, making it necessary to assess whether its findings can be extrapolated to compounded prescriptions issued in different formats, since errors or missing information in the prescription represent a potential risk to the patient (6,7).
The compounding process is a complex and manual one, involving multiple individuals and steps (2), making it particularly prone to errors (7,8). Prescription is the element that initiates the compounding process and is one of the main sources of error (5–9), and the absence of any of the legally required information can compromise the safety or efficacy of the medication (2,5–9). To reduce the frequency of errors, the use of electronic systems has been proposed (6,7,9); however, these systems still have room for improvement.
The main objective of this study is to conduct a descriptive analysis of compounded prescriptions in Spain. Secondary objectives include evaluating the prevalence of errors in these prescriptions (according to the format used: paper vs. electronic), analyzing whether there are significant differences in the number of errors per prescription based on the Autonomous Community from which it was issued, and determining which formats present more frequent errors.
MATERIAL AND METHODS
A retrospective, descriptive observational study was carried out on prescriptions collected from September 1 to November 30, 2023, at a third-party compounding pharmacy in Madrid. These prescriptions originated from compounding requests made by other pharmacies and from patients who visited this pharmacy requesting compounded medications for human use; veterinary prescriptions were excluded.
Patient and requesting pharmacy data were anonymized in the order they were collected, starting with the value 1 and assigning sequential numbers accordingly. The included data were not used for any purpose other than the research proposed in this article.
Data Extraction and Collection
Prescriptions were obtained from two sources: patients who visited the compounding pharmacy directly and third-party pharmacies that requested the preparation of compounded medications through a website. On this platform, a series of data are entered along with a copy of the medical prescription. From the information provided on the website, only the following are considered: the name of the requesting pharmacy owner, the patient’s name, and the attached prescription. All other data are disregarded.
To avoid bias, data collection for prescriptions submitted by walk-in patients was carried out by pharmacy staff who were not involved in the study. These employees applied the same screening criteria used by the requesting pharmacies.
From each prescription, the following data were extracted: the origin pharmacy and its corresponding Autonomous Community (CCAA), and the presence or absence of the mandatory information as stipulated by Royal Decree 1718/2010 (excluding the prescriber’s professional qualification, as it is not clearly defined in the regulation and does not provide relevant information). To enable grouping, additional data were recorded: the originating CCAA, the request date, the medical specialty to which the compounded medication corresponds, whether or not it is reimbursable, and coded identifiers for the pharmacy and patient names. In cases where the prescribed formulation did not clearly fall under a specific medical specialty (due to its mechanism of action or indication), it was grouped under a general category labeled “Family and Community Medicine.” Given the wide range of specialties, those with a frequency below 1% were grouped under the category “Other.”
Prescriptions were classified by the format used: paper or electronic. Paper prescriptions were further categorized by source: National Health System (SNS), mutual funds, or private entities. Electronic prescriptions were classified by origin: SNS or private sector. Additionally, for electronic prescriptions, the software used for processing was identified (e.g., Farmatic, Nixfarma, Farmanager, Bitfarma, Unycop, Sigefar, REMPE, Nodofarma, or electronic prescription receipts).
Materials
The statistical analyses were performed using IBM SPSS v24 and Excel 2013..
Descriptive Analysis
A descriptive analysis was carried out for the following: the total number of prescriptions obtained, the number of prescriptions per day, and the number of patients corresponding to the received prescriptions (reporting measures of central tendency). Subsequently, after grouping the data by Autonomous Community (CCAA), the number of pharmacies and prescriptions per CCAA was described.
A frequency analysis was conducted for medical specialties, reimbursable formulations, and the format used (paper or electronic), both overall and segmented by CCAA. In the segmentation of specialties by CCAA, only those with a frequency greater than 50 were shown.
Error Analysis
The frequency of errors was determined and then classified based on their origin into the following categories: Patient data (patient name, year of birth, and DNI or equivalent). Prescriber data (prescriber name, contact information, professional address, license number, and signature), Medication data (active ingredient, dosage, route of administration, pharmaceutical form, dosage regimen, treatment duration, and quantity to be prepared), and Other (prescription date and number of medications per prescription).
The analysis does not take into account the presence or absence of optional elements such as the expected date of dispensing, dispensing order number, or the type of recipient.
From the errors identified, the number of legally dispensable prescriptions was determined, as well as the average number of errors per CCAA. An ANOVA with Bonferroni correction and Spearman correlation was used to assess whether there were statistically significant differences between Autonomous Communities.
A cumulative variable was created to account for the absence of legally required data. An ANOVA with Bonferroni correction was then conducted to compare the mean number of errors according to the prescription format. First, the mean number of errors was compared between electronic and paper prescriptions, and subsequently, the different types of electronic and paper formats were analyzed in greater detail.
RESULTS
1. Sample Description
A total of 6,694 prescriptions were collected, with a mean of 73.56 prescriptions per day (SE = 0.49; 95% CI; median = 89; σ = 40.25), corresponding to a total of 4,241 patients. The average was 1.59 prescriptions per patient (SE = 0.02; 95% CI; median = 1; σ = 1.28), with a maximum of 26 prescriptions per patient and a minimum of 1.
When segmenting these prescriptions by Autonomous Community (CCAA) and number of requesting pharmacies, the data are presented in Table 1.
Table 1. Distribution and origin of received prescriptions. Abbreviations: CLM (Castilla-La Mancha) and CyL (Castilla y León).

Of the prescriptions obtained, those belonging to medical specialties with frequencies below 1% were grouped into the category “Other,” and 134 prescriptions (2%) with an “Unknown specialty” were excluded. Of the remaining prescriptions (see Figure 1), 1,916 prescriptions (29.21%) corresponded to dermatology, 109 (1.66%) to stomatology, 333 (5.08%) to gastroenterology, 184 (2.80%) to gynecology, 216 (3.29%) to family and community medicine, 3,322 (50.64%) to ophthalmology, 305 (4.65%) to “Other,” and 175 (2.67%) to pediatrics.
Figure 1. Breakdown of obtained prescriptions by medical specialty.
When broken down by Autonomous Community (CCAA), those with a frequency greater than 50 (accounting for 97.07% of the prescriptions) are shown in Figure 2.
Figure 2. Distribution of specialties by Autonomous Community (CCAA). Abbreviations: Desc (Unknown), Derm (Dermatology), Estom (Stomatology), Gastro (Gastroenterology), Gine (Gynecology), MFyC (Family and Community Medicine), Oftal (Ophthalmology), CLM (Castilla-La Mancha), and CyL (Castilla y León).
Of the total prescriptions collected, 2,947 (44.02%) correspond to non-reimbursable prescriptions, and 3,747 (55.98%) to prescriptions reimbursable by the Social Security system. When distributed by Autonomous Community (CCAA), the data are shown in Table 2.
Table 2. Distribution of prescriptions according to Social Security reimbursement. Abbreviations: CLM (Castilla-La Mancha) and CyL (Castilla y León).

Analyzing the format used, 3,768 prescriptions (56.29%) were issued on paper, while 2,926 (43.71%) were issued electronically. When broken down further, it was determined that:
- 2,433 prescriptions (36.35%) were issued using the SNS electronic system,
- 1,789 (26.73%) were SNS paper prescriptions,
- 246 (3.67%) were paper prescriptions from mutual insurance entities,
- 493 (7.36%) were electronic prescriptions from private sources, and
- 1,733 (25.89%) were private paper prescriptions.
When segmenting the frequency of electronic prescription use by CCAA, the results are shown in Table 3.
Table 3. Frequency of electronic format use by Autonomous Community (CCAA). Abbreviations: CLM (Castilla-La Mancha) and CyL (Castilla y León).

Of the 2,926 electronic prescriptions, only 2,778 (94.92%) could be classified according to the software used, while the remaining 5.08% corresponded to receipts, medication summaries, or other non-standard formats. The distribution of identifiable electronic prescribing systems by Autonomous Community (CCAA) is detailed in Table 4.1 and Table 4.2.
Table 4.1. Breakdown of electronic systems used by Autonomous Community (CCAA). Abbreviations: CLM (Castilla-La Mancha) and CyL (Castilla y León).

Table 4.2. Continuation of Table 4.1.

2. Error Analysis
From the total of 6,694 prescriptions obtained, a total of 50,138 errors were identified, with a mean of 7.49 errors per prescription (SE = 0.04, 95% CI, median = 8, σ = 2.88). Among these errors, 7,728 (15.42%) were due to missing patient information (see Table 5.1 for breakdown), 21,854 (43.61%) to missing prescriber information (see Table 5.2), 15,396 (30.72%) to missing formulation data (see Table 5.3), and 5,133 (10.24%) to either missing prescription dates or the inclusion of more than one medication per prescription (see Table 5.4).
Table 5.1. Frequency of Missing Patient Information. Includes absence of patient name, year of birth, and ID number or equivalent.
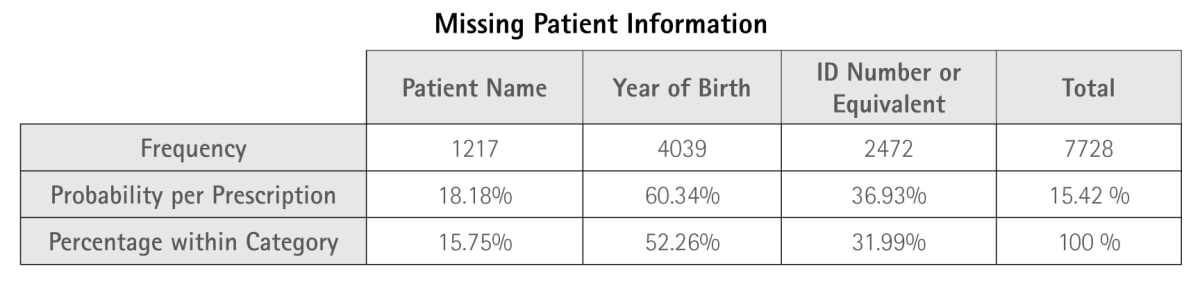
Table 5.2. Frequency of Missing Prescriber Information. Includes absence of physician’s name, contact information, professional address, medical license number, and physician’s signature.

Table 5.3. Frequency of Missing Formulation Information. Includes absence of active ingredient (AI), dosage, route of administration, pharmaceutical form (PF), posology, treatment duration, and quantity.
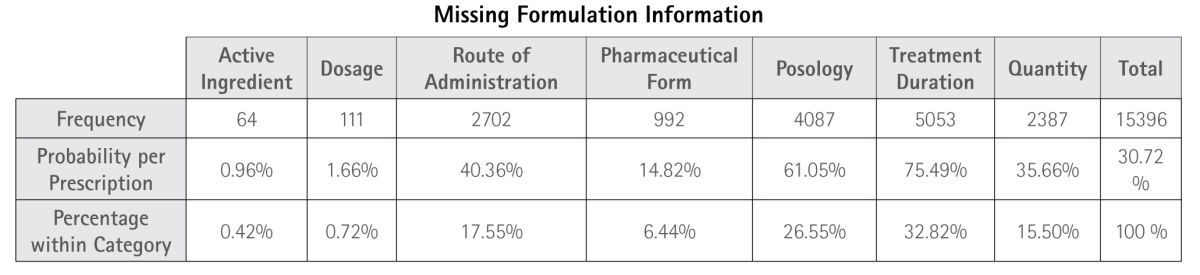
Table 5.4. Frequency of Missing Prescription Date and Presence of More Than One Medication per Prescription.

Of the 17 elements required by Royal Decree 1718/2010, none of the prescriptions met all the legal criteria to be considered dispensable. A normal distribution of errors was observed (Kolmogorov–Smirnov test, p > 0.001) (see Figure 3).
Figure 3. Distribution of Errors Based on the Absence of Elements Required by Royal Decree 1718/2010.
When segmenting the error analysis by Autonomous Communities, Table 6 presents the measures of central tendency, with their graphical representation shown in Figure 4. The mean number of errors between CCAAs differs significantly (ANOVA, p < 0.001), with a moderate Spearman correlation (p < 0.001, ρ = 0.47) and an effect size of η² = 0.29.
Table 6. Measures of Central Tendency for the Number of Errors per Prescription by Autonomous Community

Figure 4. Mean Number of Errors per Prescription in Each Autonomous Community.
Analyzing the errors based on the type of prescription medium—electronic or paper—an ANOVA was performed, revealing that the mean number of errors for paper prescriptions was 5.9 (σ = 2.89, SE = 0.04), while for electronic prescriptions it was 9.52 (σ = 2.12, SE = 0.03), with a statistically significant difference (p < 0.001). This finding is supported by a Spearman correlation (p < 0.001, ρ = 0.64) and an Eta squared association (η² = 0.39).
When segmenting by whether the prescription originated from the National Health System (SNS) or private sources, and by type of medium (see Figure 5), the following mean error rates were observed: SNS electronic prescriptions 10.26 (SE = 0.02, σ = 1.06); SNS paper prescriptions 5.27 (SE = 0.05, σ = 1.94); Mutual fund paper prescriptions 7.78 (SE = 0.14, σ = 2.25); Private electronic prescriptions 5.89 (SE = 0.10, σ = 2.27); Private paper prescriptions 6.29 (SE = 0.06, σ = 2.53). The differences in means were statistically significant according to ANOVA (p < 0.001), and post hoc Bonferroni correction confirmed significant differences between groups (p < 0.001).
Figure 5. Representation of the Mean Number of Errors by Type of Prescription Medium.
Breaking down the errors by electronic systems used within the National Health System (SNS), the following mean error rates were observed: Farmatic 10.19 (SE = 0.02, σ = 0.84), Nixfarma 10.10 (SE = 0.052, σ = 0.93), another unspecified system 10.10 (SE = 0.05, σ = 0.92), Farmanager 11.06 (SE = 0.05, σ = 0.71), Bitfarma 11.46 (SE = 0.12, σ = 1.03), Unycop 10.56 (SE = 0.11, σ = 0.95), and SNS electronic prescription printouts 9.90 (SE = 0.96, σ = 0.06). The Sigefar system was excluded from the analysis due to having only three cases. An ANOVA comparing these means revealed a statistically significant difference (p < 0.001). However, post hoc Bonferroni correction indicated that: Farmatic’s mean was significantly different only from Farmanager and Bitfarma. Nixfarma’s mean differed significantly from those of Farmanager, Bitfarma, and the SNS electronic prescription printouts. Farmanager’s and Bitfarma’s means were significantly different from the SNS printout mean.
Regarding private electronic systems, statistically significant differences were also found between all systems (p < 0.001), with the following mean error rates: REMPE 5.07 (SE = 0.13, σ = 2.18); Nodofarma 6.22 (SE = 0.22, σ = 2.16); Private electronic prescription printouts 7.77 (SE = 0.11, σ = 1.11)
Focusing specifically on the printouts from SNS and private electronic prescription systems, a statistically significant difference in their means was observed (p < 0.001).
DISCUSSION
The primary service area of the pharmacy from which the data were obtained includes the regions of CyL, CLM and Madrid, accounting for 93.05% of the prescriptions. Ophthalmology was the most frequently represented specialty, comprising 49.63% of prescriptions, which contributed to a mean of 1.59 prescriptions per patient over the three-month period. Although the study period is not sufficient to draw definitive conclusions, these findings suggest a considerable proportion of patients may require compounded medications for prolonged treatment durations.
Given that the majority of prescriptions originated from Madrid, this distribution is considered to closely reflect real-world patterns, albeit with a likely overestimation of sterile formulations. This is attributable to the presence of seven pharmacies in the region authorized to prepare sterile compounded medications for third parties (10). Within this context, the distribution by medical specialty continues to show a predominance of ophthalmology (36.49%), followed closely by dermatology (36.20%), and to a lesser extent by gastroenterology (6.86%), family and community medicine (3.95%), gynecology (3.8%), and pediatrics (3.65%). It is likely that the actual volume of dermatological formulations surpasses that of ophthalmology, the latter being potentially overrepresented due to the data originating from one of the few pharmacies in Spain authorized to compound sterile formulations.
Despite the apparent parity between reimbursed and non-reimbursed compounded medications, prescriptions originating within the National Health System (SNS) are more frequent. Notably, there is a higher prevalence of reimbursed formulations in CyL and CLM. This trend may be attributed to inter-regional price differences, the relatively low number of authorized compounding pharmacies for level 1 (non-sterile preparations) and level 2 (sterile preparations) in these regions compared to Madrid, and the potential overestimation of ophthalmic formulations. These hypotheses should be confirmed in future studies.
Regarding the types of prescription media used, contrary to expectations, there is an overall preference for paper-based prescriptions over electronic ones. This may be related to the lower average number of errors observed in paper prescriptions (mean = 5.9 errors per prescription) compared to electronic prescriptions (mean = 9.52 errors per prescription). Within the National Health System (SNS), there is a slight preference for electronic media (2,433 prescriptions) over paper (1,789 prescriptions). In contrast, in the private sector, the preference for paper-based prescriptions is more pronounced (1,733 paper vs. 493 electronic). However, with the exception of Madrid, the use of electronic prescription systems for compounded medications (CP) remains limited. These findings should be validated in future studies involving data from regions beyond CyL, CLM and Madrid.
Among the electronic prescriptions analyzed, a notable proportion (5.08%) originated from pharmacies submitting compounded medication (CP) requests using sales receipts or other non-valid or unrecognized formats, or by forwarding prescriptions that do not comply with the requirements established by Royal Decree 1718/2010 of December 17. This practice may lead to interpretation errors, compounding mistakes, and delays in the preparation of the medication, underscoring the critical role of compounding pharmacy and the need for direct communication with the prescriber.
Given the limited adoption of electronic prescription systems for compounded medications in regions outside of Madrid, only data from the Community of Madrid were considered as a reference. In this region, the most used electronic system within the National Health System (SNS) was Farmatic, followed by Nixfarma and electronic prescription printouts. In the private sector, the most frequently used system was REMPE, followed by electronic prescription printouts.
An analysis of errors per prescription reveals a high average error rate (mean = 7.49 errors per prescription), indicating a significant potential for compounding inaccuracies that may compromise the safety and efficacy of the final formulation (CP).
Among the identified errors, patient-related data represent one of the less frequent categories (15.42%); however, the frequency of missing patient names (18.18%) and years of birth (60.34%) is striking. Such omissions may lead to the selection of inappropriate excipients for a patient’s age or, more critically, to a misidentification of the intended recipient of the medication.
Within the category of prescriber-related errors, several issues stand out: the absence of the prescriber’s full name in 37.21% of cases, missing medical license numbers in 64.19%, and a lack of contact information in 84.79%. These deficiencies pose a clear risk to patient safety, as they hinder the ability to verify the prescriber’s qualifications, confirm the legitimacy of the prescription, or establish communication to make necessary clarifications or adjustments to the formulation.
With regard to formulation data, this category exhibits one of the highest rates of errors. Particularly concerning is the absence of treatment duration in 75.49% of prescriptions, missing posology in 61.05%, lack of specified quantity to be prepared in 35.67%, and the omission of the pharmaceutical form (PF) in 14.83% of cases. These omissions represent critical risks to the safety and efficacy of the compounded medication, both during its preparation and at the time of dispensing, as they hinder efforts to reinforce treatment adherence.
Among all mandatory prescription elements, the diagnosis or indication is consistently omitted. Although this parameter was not specifically evaluated in this study, its absence is especially relevant in the context of compounded medications. The presence of a diagnosis is essential to support the rationale for formulation through bibliographic references, enabling the preparation of the most appropriate compounded medication based on the diagnosis, active ingredient, dose, route of administration, pharmaceutical form, and to ensure the delivery of accurate, patient-specific information tailored to the underlying condition.
When analyzing the frequency of errors by Autonomous Community (CCAA), it is notable that the mean number of errors varies considerably depending on the region of prescription. This variability may be influenced by the type of prescription medium used and the format of paper prescriptions.
In terms of prescription medium, a significant difference is observed between paper and electronic prescriptions, as well as among the different electronic prescribing systems. Error rates are notably higher in electronic prescriptions, a finding that contradicts initial expectations. This suggests that prescriptions may not be properly completed, that electronic prescribing systems may lack adequate integration with community pharmacy management software, or that pharmacy personnel may be unable to retrieve all necessary data and forward it appropriately to the compounding pharmacy.
This issue highlights a potential risk to patient safety and should be further investigated to identify and implement strategies aimed at minimizing prescription-related errors in compounded medications.
LIMITATIONS
The data analyzed in this study were obtained from a single compounding pharmacy serving third parties, located in Madrid. As such, the prescriptions originating from other Autonomous Communities (CCAA) are likely influenced by their geographical proximity to this pharmacy, the availability of other third-party compounding pharmacies, and their respective accreditation levels for the preparation of various sterile and non-sterile pharmaceutical forms. Additionally, regional population characteristics and prescribing practices may have impacted the distribution and frequency of compounded medication (CP) requests.
Furthermore, this is a retrospective study with a limited observation period of three months. As a result, it was not possible to assess the recurrence of CP requests among patients with chronic conditions. Longer-term studies will be necessary to evaluate this aspect and to gain a more comprehensive understanding of prescribing patterns and patient needs over time.
CONCLUSIONS
Although the distribution data by medical specialty may not be generalizable, primarily due to the potential overrepresentation of sterile formulations, the findings related to prescription errors are likely applicable across all Autonomous Communities and prescription media studied, with the exception of Sigefar or other systems not included in the analysis.
Paper-based prescriptions remain highly prevalent, likely due to their lower associated error rates, as well as delays in the adoption of electronic prescription systems for compounded medications in both regional healthcare systems outside of Madrid and in the private sector.
The overall prescription error rate is alarmingly high, underscoring the urgent need for corrective measures. As proposed by Velo et al. (6), these measures should include targeted training for prescribers and pharmacists, enhanced communication between prescribers and compounding pharmacists, and improvements to prescription systems, particularly by promoting the structured use of electronic prescribing. Additionally, the explicit inclusion of diagnosis or therapeutic indication is essential to validate and optimize the prescribed treatment.
Although not the primary focus of this study, it is worth noting that many paper prescriptions from the SNS in CLM exhibit a distinct format, often including the pharmaceutical form and route of administration, as well as the active ingredient and dose. However, the stated pharmaceutical form and route are frequently incorrect or imprecise—an issue that warrants further investigation. Moreover, dosing instructions for liquid formulations are commonly expressed as quantity per 5 ml, a practice that poses a significant risk during compounding. This approach should be avoided, as it can lead to confusion and potentially serious dosing errors with harmful consequences for patients.
REFERENCES
1. Ministerio de Sanidad, Servicios Sociales e Igualdad. Real Decreto Legislativo 1/2015, de 24 de julio, por el que se aprueba el texto refundido de la Ley de garantías y uso racional de los medicamentos y productos sanitarios. [Internet]. BOE-A-2015-8343. Sec. 1, RD 1/2015 de 24 de julio jun, 2015 p. 96. Disponible en: https://www.boe.es/buscar/act.php?id=BOE-A-2015-8343
2. Busquets FB, Bagaría G, Flaqué MV. Errores de medicación en fórmulas magistrales. Boletín de Prevención de Errores de Medicación de Cataluña. 2019;17(3):1-7.
3. Grupo de Trabajo de Formulación Magistral (Consejo General de COlegios Oficiales de Farmacéuticos). La formulación magistral en España: una opción de futuro [Internet]. 2010. Disponible en: https://www.farmaceuticos.com/
4. Real Decreto 1718/2010, de 17 de diciembre, sobre receta médica y órdenes de dispensación. [Internet]. BOEA-A-2011-1013. Sec. 1, RD 1718/2010 de 17 de diciembre dic, 2010 p. 24. Disponible en: https://www.boe.es/buscar/act.php?id=BOE-A-2011-1013
5. Ayala Muñoz P, Estrada Riolobos G, Gil-Alberdi González B, Herrada Romero M, Requejo López E, Moya Rueda AP. Análisis de cumplimentación de las recetas médicas en soporte papel. FC. 20 de enero de 2021;13(1):24-31.
6. Velo GP, Minuz P. Medication errors: prescribing faults and prescription errors. Br J Clin Pharmacol. junio de 2009;67(6):624-8.
7. AMPC. Medication Errors | AMCP.org [Internet]. 2019 [citado 9 de marzo de 2025]. Disponible en: https://www.amcp.org/concepts-managed-care-pharmacy/medication-errors
8. Amorim SHBM de, Lopes LPN, Belmiro VB de S, Passos MMB dos, Monteiro MS de S de B, Junior ER, et al. Medication Errors in Compounding Pharmacy. Journal of Health Sciences. diciembre de 2021;23(4):316-22.
9. Reed-Kane D, Kittell K, Adkins J, Flocks S, Nguyen T. E-prescribing errors identified in a compounding pharmacy: a quality-improvement project. Int J Pharm Compd. 1 de enero de 2014;18(1):83-6.
10. Dirección General de Ordenación e inspección (Consejería de Sanidad de la Comunidad de Madrid). Oficinas de farmacia autorizadas para la elaboración a terceros de fórmulas magistrales y preparados oficinales. Consejería de Sanidad de la Comunidad de Madrid; 2025.
Editor: © SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria.
Copyright© SEFAC. Sociedad Española de Farmacia Clínica, Familiar y Comunitaria. This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
https://creativecommons.org/licenses/by-nc/4.0/deed.en


